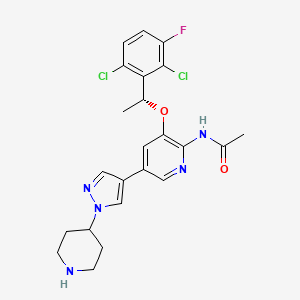
Unecritinib
- CAS 1418026-92-2
- 4T3Z98RR86
- TQ-B3101
492.4 g/mol, C23H24Cl2FN5O2
N-[3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-(1-piperidin-4-ylpyrazol-4-yl)pyridin-2-yl]acetamide
- Acetamide, N-[3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1-(4-piperidinyl)-1H-pyrazol-4-yl]-2-pyridinyl]-
- N-{3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-[1- (piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-yl}acetamide
Chia Tai Tianqing Pharmaceutical Group
Unecritinib is an orally available, small molecule inhibitor of the receptor tyrosine kinases anaplastic lymphoma kinase (ALK), C-ros oncogene 1 (ROS1) and Met (hepatocyte growth factor receptor; HGFR; c-Met), with potential antineoplastic activity. Upon oral administration,unecritinib targets, binds to and inhibits the activity of ALK, ROS1 and c-Met, which leads to the disruption of ALK-, ROS1- and c-Met-mediated signaling and the inhibition of cell growth in ALK-, ROS1- and c-Met-expressing tumor cells. ALK, ROS1 and c-Met, overexpressed or mutated in many tumor cell types, play key roles in tumor cell proliferation, survival, invasion and metastasis.
UNECRITINIB is a small molecule drug with a maximum clinical trial phase of II (across all indications) and has 3 investigational indications.
- OriginatorChia Tai Tianqing Pharmaceutical Group
- ClassAcetamides; Antineoplastics; Benzofurans; Chlorobenzenes; Esters; Ethers; Fluorobenzenes; Ketones; Morpholines; Piperidines; Pyrazoles; Pyridines; Small molecules
- Mechanism of ActionAnaplastic lymphoma kinase inhibitors; Proto-oncogene protein c-met inhibitors; ROS1 protein inhibitors
- RegisteredNon-small cell lung cancer
- No development reportedAnaplastic large cell lymphoma
- 07 Sep 2024Efficacy and adverse events data from a phase II trial in Non-small cell lung cancer presented at the 25th World Conference on Lung Cancer (WCLC-2024)
- 17 May 2024Chemical structure information added
- 17 May 2024No development reported – Phase-II for Anaplastic large cell lymphoma (In adolescents, In children, Late-stage disease, Refractory metastatic disease, Second-line therapy or greater, In adults) in China (PO)
PATENT
https://patentscope.wipo.int/search/en/WO2013041038
Example 11: Synthesis of
(R)-N-(3-(l-(2,6-dichloro-3-fluorophenyl)ethoxy)- 5-(l -(piperidin-4-yl)-lH-pyrazol-4-yl)pyridin-2-yl)acetamide (Compound 18)
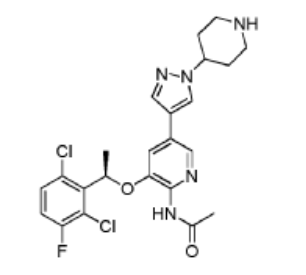
Step 1. To a solution of (R)-tert-butyl 4-(4-(6-amino-5-(l-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-3 -yl)- 1 H-pyrazol- 1 -yl)piperidine- 1 -carboxylate ( 4g, 7.27 mmol, 1.0 eq) and pyridine ( 2.3g, 29.1 mmol, 4.0 eq) in 50 ml DCM was added acetyl chloride (0.86g, 10.9 mmol, 1.5 eq) in an ice bath. The reaction mixture was stirred at room temperature for overnight. The resulting mixture was washed with H20 (3×20 mL). The organic layer was dried and concentrated. The crude product was purified on silica gel column to give (R)-tert-butyl 4-(4-(6-acetamido-5-(l-(2,6-dichloro-3-fluorophenyl)ethoxy)pyridin-3-yl)-lH-pyrazol-l-yl)piperidine-l-carboxylatel .66g (38.6% yield).
Step 2. To a solution of (R)-tert-butyl 4-(4-(6-acetamido-5-(l-(2,6-dichloro-3 -fluorophenyl)ethoxy)pyridin-3 -yl)- 1 H-pyrazol- 1 -yl)piperidine- 1 -carboxylate (500 mg, 0.84 mmol, 1.0 eq) in DCM (5 mL) was added trifluoroacetic acid (2 ml) in an ice bath. The reaction mixture was stirred at room temperature for 2 hours. The pH of the reaction mixture was adjusted to 9 by saturated bicarbonate sodium in an ice bath. The aqueous solution was extracted with ethyl acetate (3×20 mL), the combined organic layers were washed with brine, dried over (MgSC^), filtered, and concentrated. The crude product was purified by silica gel column to give (R)-N-(3 -( 1 -(2,6-dichloro-3 -fluorophenyl)ethoxy)-5-( 1 -(piperidin-4-yl)- 1 H-pyrazol-4-yl)pyridin-2-yl)acetamide 250 mg (60.2% yield).
^-NMR^DC , 400Hz): 51.88(d, J=6.4Hz, 3H), 51.90-1.94(m, 2H), 52.16-2.20(m, 2H), 52.48(s, 3H), 52.76-2.824(m, 2H), 53.25-3.28(m, 2H), 53.69-3.74(m, 1H), 54.22-4.26 (m, 1H), 56.10-6.15(m, 1H), 57.05-7.07 (m, 1H), 57.09(s, 1H), 57.30-7.33 (m, 1H), 57.59(s, 1H), 57.62(s, 1H), 58.06(s, 1H),
58.12(s, 1H). MS m/z 493 [M+l]
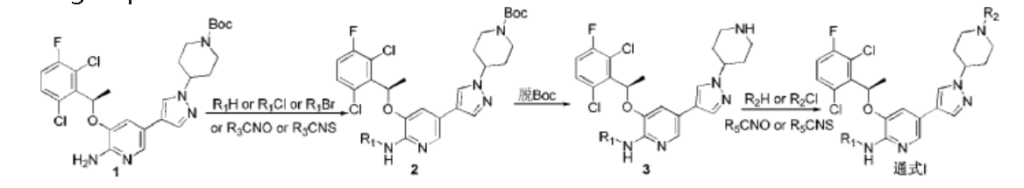
PATENT
CN102850328
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN85774618&_cid=P12-MECPSG-91316-1
SYN
European Journal of Medicinal Chemistry 291 (2025) 117643
Unecritinib, developed by Chia Tai Tianqing Pharmaceutical Group, is a novel small-molecule tyrosine kinase inhibitor. It targets c-rosoncogene 1 (ROS1), anaplastic lymphoma kinase (ALK), and c-mesen
chymal-epithelial transition factor (c-MET) kinases, exhibiting potent antitumor activity against cancers harboring these genetic alterations. In 2024, the NMPA approved Unecritinib under the brand name Anbaini for the treatment of adult patients with ROS1-positive locally advanced or metastatic non-small cell lung cancer (NSCLC). Unecritinib exerts its therapeutic effects through selective inhibition of the kinase activities of ROS1, ALK, and c-MET, which effectively disrupts the downstream signaling pathways that are crucial for the proliferation and survival of tumor cells. Consequently, this inhibition induces cell cycle arrest and apoptosis in cancer cells that express these specific targets [13]. The clinical efficacy of Unecritinib was established in a Phase II single-arm, multicenter clinical trial (NCT03750739) enrolling patients with ROS1-positive advanced NSCLC. Among 111 evaluable patients, an ORR of 80.2 % was achieved, along with a median PFS of 16.5 months. These findings underscore the robust antitumor activity of Unecritinib in this specific patient cohort. In terms of safety, Unecritinib exhibited a
favorable tolerability profile. The most frequently reported treatment-related adverse events were neutropenia, leukopenia, vomit ing, and nausea, which were predominantly of mild (Grade 1) or mod
erate (Grade 2) severity. Importantly, no dose-limiting toxicities were observed, and the maximum tolerated dose was not established, further supporting its favorable safety profile. The approval of Unecritinib represents a novel therapeutic strategy for patients with ROS1-positive NSCLC, effectively addressing a significant unmet medical need within this population [13].
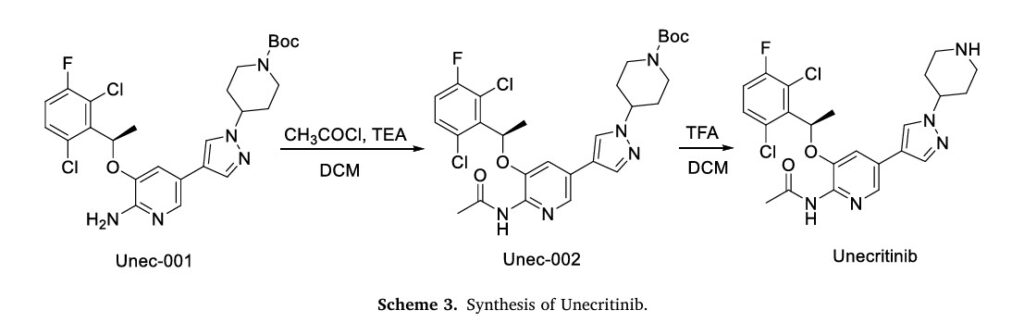
The synthesis of Unecritinib, depicted in Scheme 3, initiates with acetylation of Unec-001 to yield Unec-002, which undergoes deprotection to afford Unecritinib [14]
[13] S. Lu, H. Pan, L. Wu, Y. Yao, J. He, Y. Wang, X. Wang, Y. Fang, Z. Zhou, X. Wang,
X. Cai, Y. Yu, Z. Ma, X. Min, Z. Yang, L. Cao, H. Yang, Y. Shu, W. Zhuang, S. Cang,
J. Fang, K. Li, Z. Yu, J. Cui, Y. Zhang, M. Li, X. Wen, J. Zhang, W. Li, J. Shi, X. Xu,
D. Zhong, T. Wang, J. Zhu, Efficacy, safety and pharmacokinetics of unecritinib
(TQ-B3101) for patients with ROS1 positive advanced non-small cell lung cancer: a
phase I/II trial, Signal Transduct Target Ther 8 (2023) 249.
[14] A. Zhang, M. Geng, Y. Wang, J. Ai, X. Peng, Preparation of Pyridine Compounds as
Inhibitors of c-Met And/Or ALK Kinases, Shanghai Institute of Materia Medica,
2013 CN102850328A.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
/////////Unecritinib, Chia Tai Tianqing Pharmaceutical Group, 1418026-92-2, 4T3Z98RR86, TQ B3101, APPROVALS 2024, CHINA 2024














