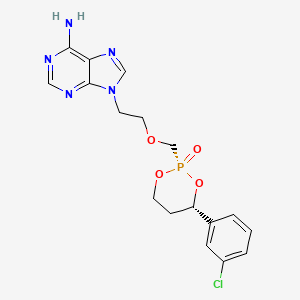
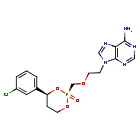
Pradefovir
WeightAverage: 423.79
Monoisotopic: 423.0863188
Chemical FormulaC17H19ClN5O4P
9-[2-[[(2R,4S)-4-(3-chlorophenyl)-2-oxo-1,3,2λ5-dioxaphosphinan-2-yl]methoxy]ethyl]purin-6-amine
2R,4S-(+)-9-(2-(4-(3-chlorophenyl)-2-oxo-1,3,2-dioxaphosphorinan-2-yl)methoxyethyl)adenine
HEPATITIS B VIRUS, APPROVALS 2024, CHINA 2024, Xi’an Xintong Pharmaceutical Research Co, Xinshumu
Pradefovir Mesylate
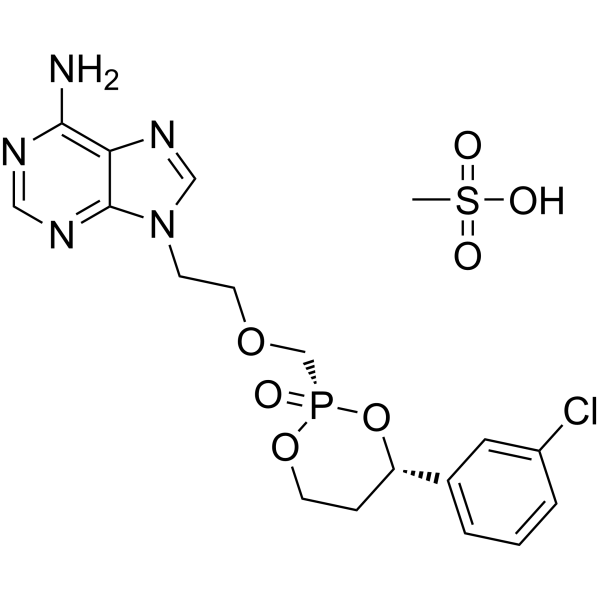
CAS No. : 625095-61-6, Remofovir mesylate
| 분자량 | 519.90 |
|---|---|
| 화학식 | C18H23ClN5O7PS |
Pradefovir is a cyclodiester antiviral prodrug with specific activity against hepatitis B virus (HBV). Pradefovir is specifically metabolized in the liver by hepatic enzymes, mainly by CYP4503A4, to adefovir. In turn, adefovir is phosphorylated by cellular kinases to its activated form adevofir diphosphate. By competing with the natural substrate dATP, the diphosphate form is incorporated into viral DNA and inhibits RNA-dependent DNA polymerase. This causes DNA chain termination and eventually results in an inhibition of HBV replication.
PAT
- Novel phosphonic acid based prodrugs of PMEA and its analoguesPublication Number: US-2003229225-A1Priority Date: 2002-05-13
- Process for Preparation of Cyclic Prodrugs of PMEA and PMPAPublication Number: US-2007203339-A1Priority Date: 2002-05-13
- Process for preparation of cyclic prodrugs of PMEA and PMPAPublication Number: US-7193081-B2Priority Date: 2002-05-13Grant Date: 2007-03-20
- Phosphonic acid based prodrugs of PMEA and its analoguesPublication Number: US-7214668-B2Priority Date: 2002-05-13Grant Date: 2007-05-08
- Lewis acid mediated synthesis of cyclic estersPublication Number: US-2005282782-A1Priority Date: 2004-06-08
- Lewis acid mediated synthesis of cyclic estersPublication Number: US-7582758-B2Priority Date: 2004-06-08Grant Date: 2009-09-01
- Process for preparation of cyclic prodrugs of pmea and pmpaPublication Number: EP-1504014-B1Priority Date: 2002-05-13Grant Date: 2015-12-02
- Salts of a phosphonic acid based prodrug of PMEAPublication Number: EP-2223927-B1Priority Date: 2002-05-13Grant Date: 2014-10-15
- Process for preparation of cyclic prodrugs of PMEA and PMPAPublication Number: US-2003225277-A1Priority Date: 2002-05-13
Syn
J. Med. Chem. 51 (2008) 666–676
SYN
https://pubs.acs.org/doi/10.1021/jm7012216
Syn
European Journal of Medicinal Chemistry 291 (2025) 117643
Pradefovir, developed by Xi’an Xintong Pharmaceutical Research Co., Ltd., is a liver-targeted nucleotide analog prodrug designed for the treatment of chronic hepatitis B virus (HBV) infection. It was approved
by the NMPA in 2024, under the brand name Xinshumu, for the treat ment of adult patients with chronic hepatitis B. Pradefovir utilizes HepDirect liver-targeting technology, allowing it to remain stable in the
gastrointestinal tract and bloodstream. It is specifically metabolized into its active form in the liver by the enzyme CYP3A4, leading to high hepatic concentrations and low systemic exposure. This targeted activation enhances antiviral efficacy while minimizing potential side effects on other organs. The clinical efficacy of pradefovir was demonstrated in a Phase III randomized, double-blind, positive-controlled trial (NCT04543565) involving patients with chronic hepatitis B. Participants were randomized to receive pradefovir or tenofovir disoproxil fumarate (TDF) in a 2:1 ratio, with a treatment duration of 96 weeks
followed by a 48-week open-label extension. Interim analysis conducted after 48 weeks showed that pradefovir achieved comparable reductions in HBV DNA levels to TDF, with a favorable safety profile. Regarding safety, pradefovir exhibited a favorable profile [90]. The total occurrence rate of adverse events was similar in both the pradefovir and TDF groups. Nevertheless, the incidence of drug-related adverse events was notably lower in the pradefovir group. Significantly, compared to the typical concerns associated with nucleotide analogs, pradefovir showed a diminished influence on renal function and bone mineral density. This is a crucial aspect considering the known side-effects of nucleotide an alogs in clinical applications, where issues related to kidney function and bone health often pose challenges. Pradefovir, in contrast, appears to have a more favorable safety profile in these regards, which could potentially offer significant advantages in long-term treatment scenarios
[91]. Additionally, it had minimal effects on lipid profiles, suggesting a lower potential risk for cardiovascular events during long-term therapy.
The approval of pradefovir mesylate offers a new therapeutic option for adult patients with chronic hepatitis B, providing effective antiviral activity with an improved safety profile, particularly concerning renal and bone health [92,93].
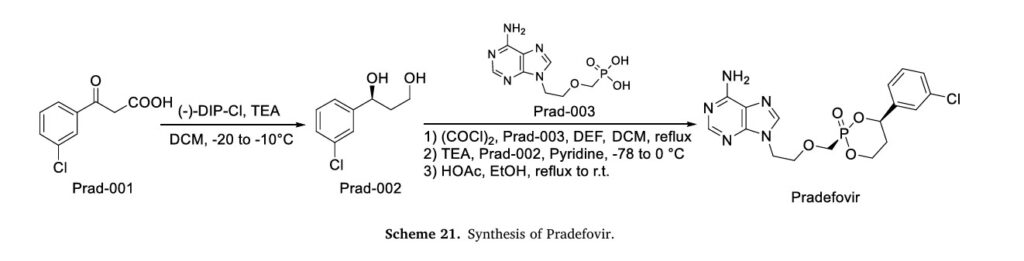
The synthetic route of Pradefovir Mesylate is shown in Scheme 21 [93]. The route commences with a stereoselective reduction of Prad-001 employing ( )-DIP-Cl, affording Prad-002. Subsequent acid-catalyzed cyclodehydration between the hydroxyl groups of Prad-002 and Prad-003 generates Pradefovir, followed by mesylate salt formation to complete the synthesis.
90-93
[90] Y. Gao, F. Kong, X. Song, J. Shang, L. Yao, J. Xia, Y. Peng, W. Liu, H. Gong, M. Mu,
H. Cui, T. Han, W. Chen, X. Wu, Y. Yang, X. Yan, Z. Jin, P. Wang, Q. Zhu, L. Chen,
C. Zhao, D. Zhang, W. Jin, D. Wang, X. Wen, C. Liu, J. Jia, Q. Mao, Y. Ding, X. Jin,
Z. Zhang, Q. Mao, G. Li, J. Niu, Pradefovir treatment in patients with chronic
hepatitis B: week 24 results from a multicenter, double-blind, randomized,
noninferiority, phase 2 trial, Clin. Infect. Dis. 74 (2022) 1925–1932.
[91] Y. Ding, H. Zhang, X. Li, C. Li, G. Chen, H. Chen, M. Wu, J. Niu, Safety,
pharmacokinetics and pharmacogenetics of a single ascending dose of pradefovir, a
novel liver-targeting, anti-hepatitis B virus drug, in healthy Chinese subjects,
Hepatol Int 11 (2017) 390–400.
[92] H. Zhang, M. Wu, X. Zhu, C. Li, X. Li, W. Jin, D. Zhang, H. Chen, C. Liu, Y. Ding,
J. Niu, J. Liu, Safety, efficacy, and pharmacokinetics of pradefovir for the
treatment of chronic hepatitis B infection, Antiviral Res 174 (2020) 104693.
[93] K.R. Reddy, M.C. Matelich, B.G. Ugarkar, J.E. G´omez-Galeno, J. DaRe, K. Ollis,
Z. Sun, W. Craigo, T.J. Colby, J.M. Fujitaki, S.H. Boyer, P.D. van Poelje, M.D. Erion,
Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis
B, J. Med. Chem. 51 (2008) 666–676.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
//////////Pradefovir, HEPATITIS B VIRUS, APPROVALS 2024, CHINA 2024, Xi’an Xintong Pharmaceutical Research Co, Xinshumu, Pradefovir Mesylate, Remofovir, 625095-60-5, GZE85Q9Q61, DTXSID10870372, MB 6866, MB-06866, ICN2001-3














