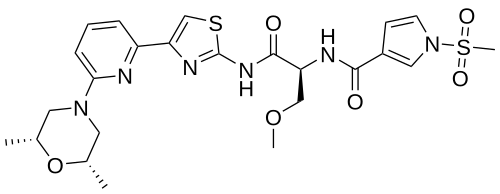
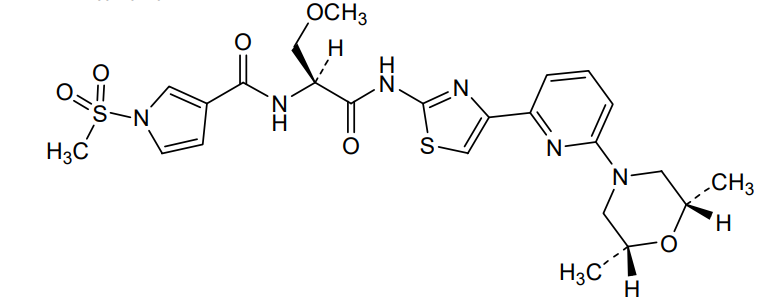
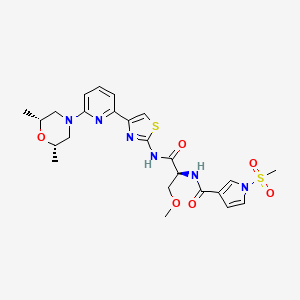
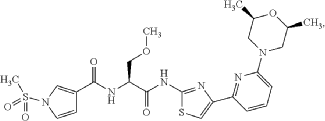
Camibirstat
CAS 2671128-05-3
N-{(2S)-1-[(4-{6-[(2R,6S)-2,6-dimethylmorpholin-4-yl]pyridin-2-yl}-1,3-thiazol-2-yl)amino]-3-methoxy-1-oxopropan-2-yl}-1-(methanesulfonyl)-1H-pyrrole-3-carboxamide
ATPase inhibitor, antineoplastic
MW C24H30N6O6S2 MF 562.7 g/mol
- 1H-Pyrrole-3-carboxamide, N-((1S)-2-((4-(6-((2R,6S)-2,6-dimethyl-4-morpholinyl)-2-pyridinyl)-2-thiazolyl)amino)-1-(methoxymethyl)-2-oxoethyl)-1-(methylsulfonyl)-
- N-[(2S)-1-[[4-[6-[(2S,6R)-2,6-dimethylmorpholin-4-yl]pyridin-2-yl]-1,3-thiazol-2-yl]amino]-3-methoxy-1-oxopropan-2-yl]-1-methylsulfonylpyrrole-3-carboxamide
- FHD 286
Camibirstat is an investigational new drug that is being evaluated for the treatment of cancer. It is a small molecule that acts as a selective inhibitor of SMARCA2 and SMARCA4, which are key components of the SWI/SNF chromatin remodeling complex.[1]
It is being developed by Foghorn Therapeutics.[2]
Camibirstat is an orally bioavailable, allosteric, small molecule inhibitor of transcription activator BRG1 (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 4; SMARCA4) and BRM (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 2; SMARCA2), with potential antineoplastic activity. Upon oral administration, camibirstat targets, binds to, and inhibits the activity of BRG1 and/or BRM, the primary ATPase components and mutually exclusive subunits of the BRG1/BRM-associated factor (BAF) complexes. This may lead to the inhibition of the SWI/SNF chromatin remodeling complex, disrupt chromatin remodeling and gene expression, and result in the downregulation of oncogenic pathways and the inhibition of tumor cell proliferation. BAF is an important regulator of transcriptional programs and gene expression. Mutations in BAF or its transcription factor partners are found in certain diseases including cancers.
PAT
- Compounds and uses thereofPublication Number: US-2024101550-A1Priority Date: 2020-01-29
- Compounds and their usesPublication Number: JP-7561195-B2Priority Date: 2020-01-29Grant Date: 2024-10-03
- Compounds and uses thereofPublication Number: TW-I859406-BPriority Date: 2020-01-29Grant Date: 2024-10-21
- Compounds and uses thereofPublication Number: IL-295100-APriority Date: 2020-01-29
- Compounds and uses thereofPublication Number: KR-20220133258-APriority Date: 2020-01-29
- Compounds and uses thereofPublication Number: US-11485732-B2Priority Date: 2020-01-29Grant Date: 2022-11-01
- Compounds and uses thereofPublication Number: JP-2023512039-APriority Date: 2020-01-29
- Compounds and uses thereofPublication Number: US-2023129003-A1Priority Date: 2020-01-29
- Compounds and uses thereofPublication Number: WO-2021155262-A1Priority Date: 2020-01-29
- Compounds and uses thereofPublication Number: TW-202136252-APriority Date: 2020-01-29
- Compounds and uses thereofPublication Number: AU-2021213811-A1Priority Date: 2020-01-29
- Compound and use thereofPublication Number: CN-115023226-APriority Date: 2020-01-29
- Compounds and uses thereofPublication Number: EP-4096664-A1Priority Date: 2020-01-29
- Methods of treating cancersPublication Number: WO-2021236080-A1Priority Date: 2020-05-20
- Methods of treating cancersPublication Number: EP-4153176-A1Priority Date: 2020-05-20
- ways to treat cancerPublication Number: CN-115867314-APriority Date: 2020-05-20
- how to treat cancerPublication Number: JP-2023535124-APriority Date: 2020-05-20
- Compounds and uses thereofPublication Number: US-2021230154-A1Priority Date: 2020-01-29
- Treatment options with inhibitors of BRG1 and BRM enzyme activityPublication Number: CN-117337179-APriority Date: 2021-03-19
- Therapeutic regimens of an inhibitor of the enzymatic activity of brg1 and brmPublication Number: EP-4308124-A1Priority Date: 2021-03-19
- Treatment regimens for inhibitors of BRG1 and BRM enzymatic activityPublication Number: JP-2024511383-APriority Date: 2021-03-19
- Therapeutic regimens of an inhibitor of the enzymatic activity of brg1 and brmPublication Number: US-2024189318-A1Priority Date: 2021-03-19
- Methods of treating cancersPublication Number: US-2022079940-A1Priority Date: 2020-05-20
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=US331910582&_cid=P11-MG1TKU-39131-1
Example 1. Preparation of N—((S)-1-((4-(6-(cis-2,6-dimethylmorpholino)pyridin-2-yl)thiazol-2-yl)amino)-3-methoxy-1-oxopropan-2-yl)-1-(methylsulfonyl)-1H-pyrrole-3-carboxamide
| N—((S)-1-((4-(6-(cis-2,6-dimethylmorpholino)pyridin-2-yl)thiazol-2-yl)amino)-3-methoxy-1-oxopropan-2-yl)-1-(methylsulfonyl)-1H-pyrrole-3-carboxamide was synthesized as shown in Scheme 1 below. |
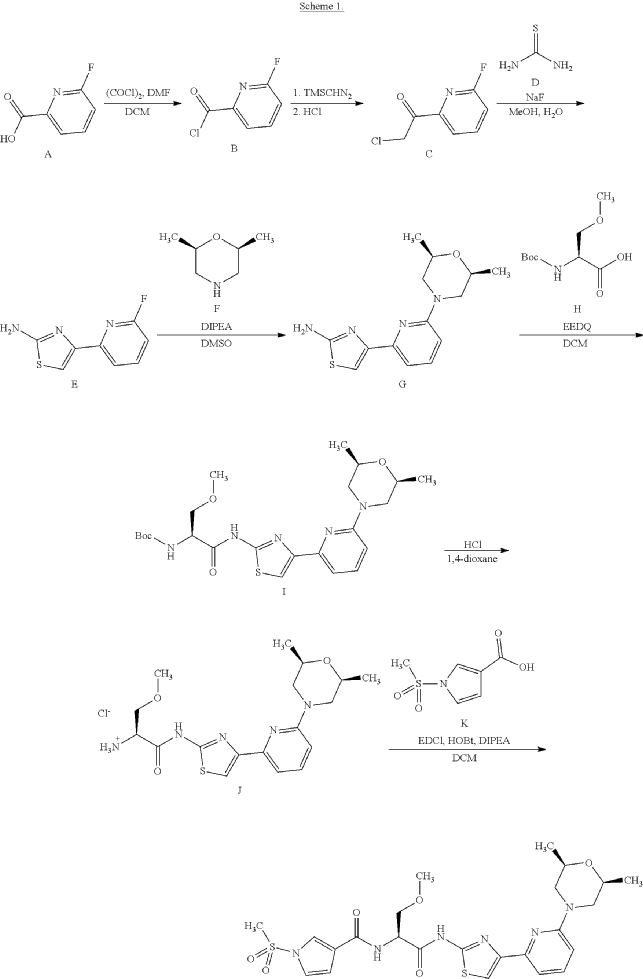
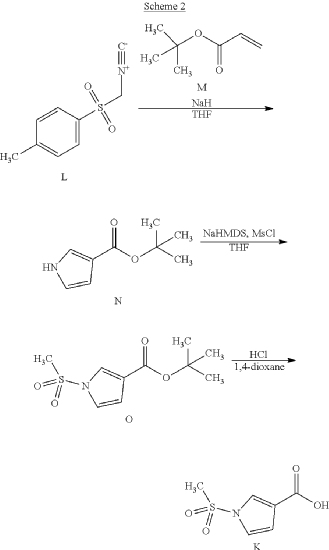
Step 7: Preparation of N—((S)-1-((4-(6-(cis-2,6-dimethylmorpholino)pyridin-2-yl)thiazol-2-yl)amino)-3-methoxy-1-oxopropan-2-O-1-(methylsulfonyl)-1H-pyrrole-3-carboxamide



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | FHD286 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2671128-05-3 |
| PubChem CID | 156818030 |
| ChemSpider | 115010237 |
| UNII | QHA5XLA4SA |
| ChEMBL | ChEMBL5095181 |
| Chemical and physical data | |
| Formula | C24H30N6O6S2 |
| Molar mass | 562.66 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- Yu L, Wu D (July 2024). “SMARCA2 and SMARCA4 Participate in DNA Damage Repair”. Frontiers in Bioscience (Landmark Edition). 29 (7): 262. doi:10.31083/j.fbl2907262. PMID 39082357.
- “Camibirstat”. PatSnap.
/////////////Camibirstat, ATPase inhibitor, antineoplastic, Foghorn Therapeutics, FHD 286














