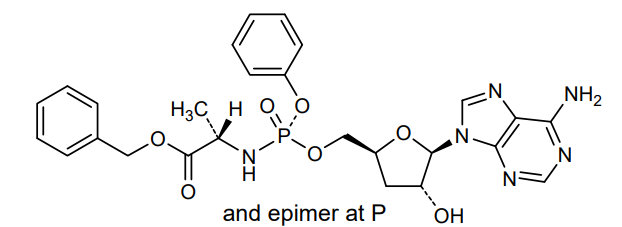
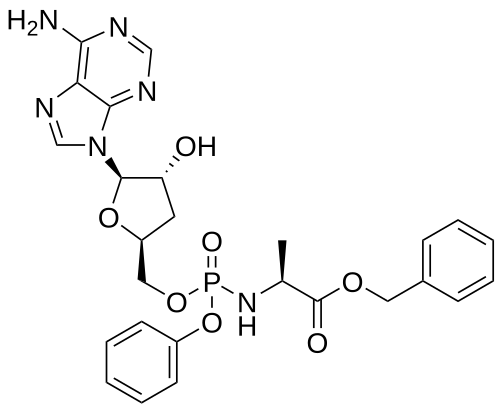
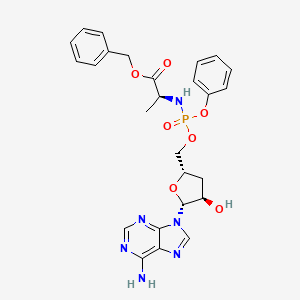

Fosdesdenosine sipalabenamide
CAS 2348493-39-8
MF C26H29N6O7P, MW=568.5 g/mol
benzyl N-(P-ambo-3′-deoxy-OP-phenyl-5′-adenylyl)-Lalaninate
benzyl (2S)-2-[[[(2S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate
3′-Deoxyadenosine 5′-O-phenyl-(benzoxy-L-alaninyl)-phosphatenucleoside analogue, antineoplastic, NUC 7738, Y7BFN2M72F
Fosdesdenosine sipalabenamide is an investigational new drug that is being evaluated for the treatment of advanced solid tumors and lymphoma.[1] This compound is a phosphoramidate derivative of cordycepin (3′-deoxyadenosine), an adenosine analog originally isolated from the fungus Cordyceps.[2][3] As a nucleoside analog with potential antineoplastic properties, Fosdesdenosine sipalabenamide is designed to inhibit RNA synthesis and act as an RNA inhibitor.[1] The drug is being developed by NuCana Plc.[1]
Fosdesdenosine Sipalabenamide is a phosphoramidate derivative of the monophosphate form of cordycepin (3′-deoxyadenosine; 3′-dA), an adenosine derivative first isolated from Cordyceps sinensis, with potential antineoplastic, antioxidant, and anti-inflammatory activities. Upon administration and cellular uptake of fosdesdenosine sipalabenamide by passive diffusion, cordycepin monophosphate (3′-dAMP) is converted into its active anti-cancer metabolite 3′-deoxyadenosine triphosphate (3′-dATP). 3′-dATP functions as a ribonucleoside analogue and competes with ATP during transcription. Therefore, this agent causes RNA synthesis inhibition, inhibits cellular proliferation, and induces apoptosis. Also, 3′-dAMP activates AMP-activated protein kinase (AMPK) and reduces mammalian target of rapamycin (mTOR) signaling. This prevents the hyperphosphorylation of the translation repressor protein 4E-BP1. This results in the induction of tumor cell apoptosis and a decrease in tumor cell proliferation. mTOR, a serine/threonine kinase belonging to the phosphatidylinositol 3-kinase (PI3K)-related kinase (PIKK) family, plays an important role in the PI3K/AKT/mTOR signaling pathway that regulates cell growth and proliferation, and its expression or activity is frequently dysregulated in human cancers. Compared to cordycepin alone, the addition of the phosphoramidate moiety may overcome cancer resistance and allow for greater cytotoxicity as fosdesdenosine sipalabenamide does not require a nucleoside transporter for cellular uptake, is independent of enzymatic activation by adenosine kinase (AK) and is not susceptible to enzymatic degradation by adenosine deaminase (ADA). Altogether, this may help overcome cancer resistance to cordycepin.
SYN
Publication Name: Journal of Medicinal Chemistry
Publication Date: 2022-11-23
PMCID: PMC9743095
PMID: 36417756
DOI: 10.1021/acs.jmedchem.2c01348
Rp)- and (Sp)-3′-Deoxyadenosine 5′-O-phenyl-(benzoxy-l-alaninyl)-phosphate (7a)
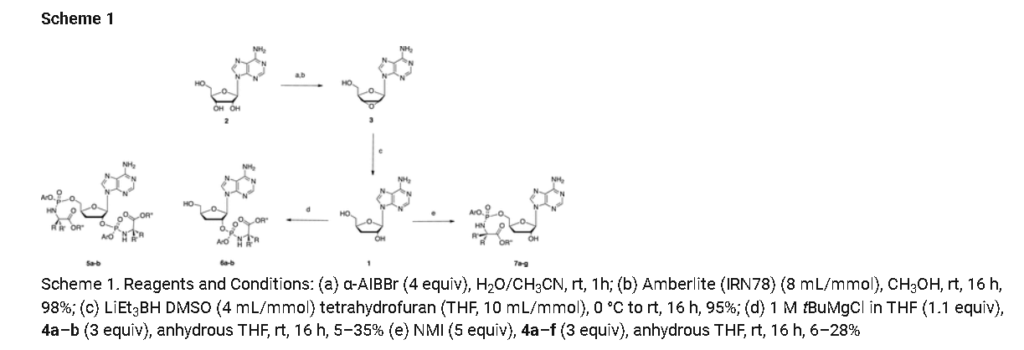
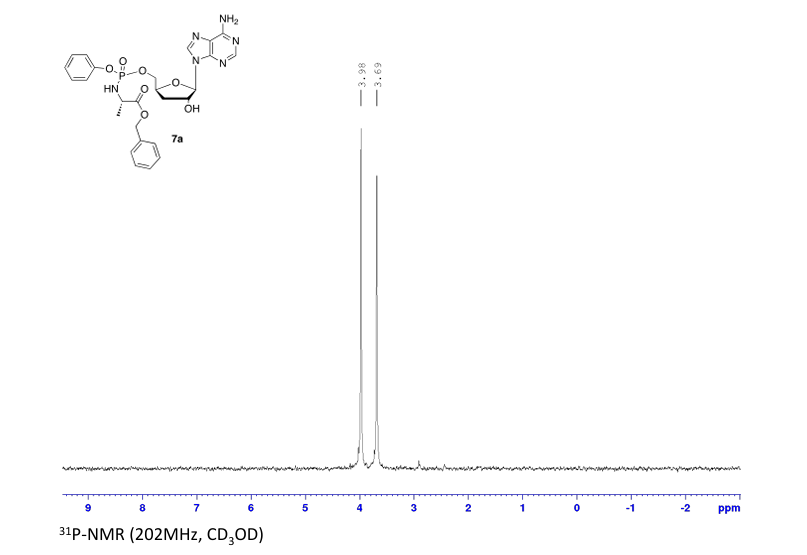
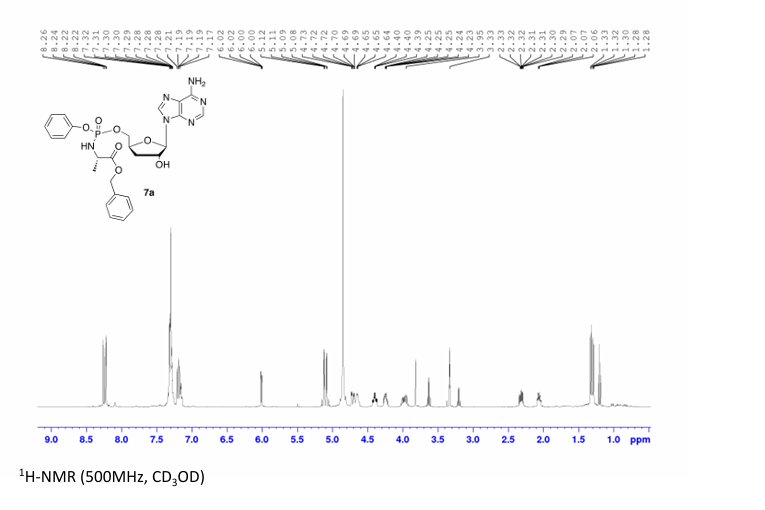
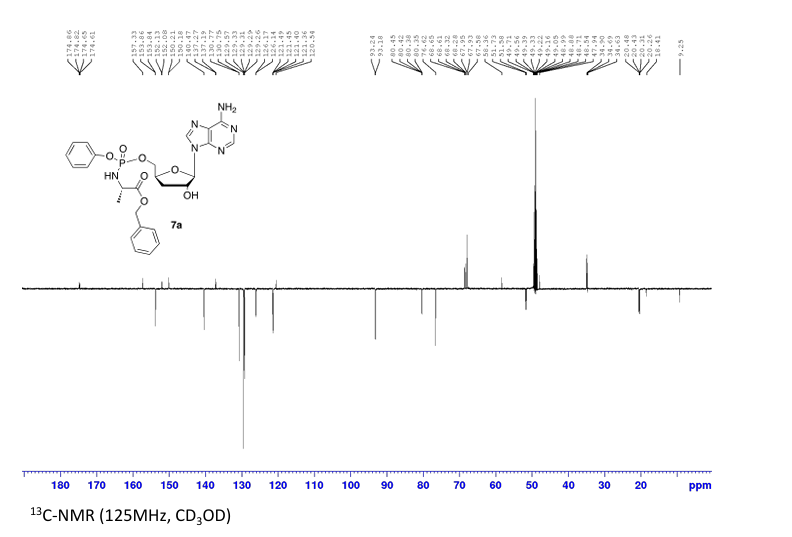
Prepared according to general procedure C using 3′-deoxyadenosine (1) (0.05 g, 0.20 mmol) in anhydrous THF (4 mL), N-methyl imidazole (0.080 μL, 1.0 mmol), and phenyl(benzyloxy-l-alaninyl) phosphorochloridate (4a) (0.021 g, 0.6 mmol) in THF (2.4 mL) Purification by Biotage Isolera One (cartridge SNAP 25 g, 25 mL/min, CH3OH/CH2Cl2 1–8% 10 CV, 8% 5 CV) and preparative TLC (1000 μM, eluent system CH3OH/CH2Cl2 5/95) afforded the title compound 7a as a white solid (0.032 g, 28%). 31P NMR (202 MHz, CD3OD) δP 3.91, 3.73. 1H NMR (500 MHz, CDCl3) δH 8.26 (s, 0.5H, H-8), 8.24 (s, 0.5H, H-8), 8.22 (s, 0.5H, H-2), 8.21 (s, 0.5H, H-2), 7.34–7.25 (m, 7H, Ar), 7.21–7.13 (m, 3H, Ar), 6.01 (d, J = 1.5 Hz, 0.5H, H-1′), 6.00 (d, J = 1.5 Hz, 0.5H, H-1′), 5.15–5.04 (m, 2H, CH2Ph), 4.73–4.63 (m, 2H, H-2′, H-4′), 4.43–4.35 (m, 1H, H-5′), 4.27–4.20 (m, 1H, H-5′), 4.03–3.91 (m, 1H, CHCH3), 2.35–2.28 (m, 1H, H-3′), 2.09–2.02 (m, 1H, H-3′), 1.32 (d, J = 7.4 Hz, 1.5 H, CHCH3), 1.28 (d, J = 7.4 Hz, 1.5 H, CHCH3). 13C NMR (125 MHz, CD3OD) δC 174.84 (d, 3JC-P = 4.5 Hz, C=O), 174.63 (d, 3JC-P = 4.5 Hz, C═O), 157.32 (C-6), 157.31 (C-6), 153.86 (C-2), 153.84 (C-2), 152.13 (C-4), 152.07 (C-4), 150.20 (C-Ar), 150.18 (C-Ar), 140.47 (C-8), 137.26 (C-Ar), 137.19 (C-Ar), 130.76 (CH-Ar), 130.74 (CH-Ar), 129.57 (CH-Ar), 129.32 (CH-Ar), 129.31 (CH-Ar), 129.29 (CH-Ar), 129.26 (CH-Ar), 126.16 (CH-Ar), 126.14 (CH-Ar), 121.46 (d, 3JC-P = 4.7 Hz, CH-Ar), 121.38 (d, 3JC-P = 4.7 Hz, CH-Ar) 120.54 (C-5), 120.53 (C-5), 93.24 (C-1′), 93.18 (C-1′), 80.43 (d, 3JC-P = 3.6 Hz, C-4′), 80.36 (d, 3JC-P = 3.6 Hz, C-4′), 76.62 (C-2′), 68.62 (d, 2JC-P = 5.3 Hz, C-5′), 68.30 (d, 2JC-P = 5.3 Hz, C-5′), 67.95 (CH2Ph), 67.92 (CH2Ph), 51.74 (CHCH3), 51.60 (CHCH3), 34.91 (C-3′), 34.70 (C-3′), 20.45 (d, 3JC-P = 7.0 Hz, CHCH3), 20.28 (d, 3JC-P = 7.0 Hz, CHCH3). Reversed-phase HPLC eluting with H2O/CH3CN from 100/10 to 0/100 in 30 min, F = 1 mL/min, λ = 254 nm, tR 13.56 and 13.75 min. C26H29N6O7P required m/z 568.2 [M]. MS (ES+) found m/z 569.2 [M + H]+, 591.2 [M + Na]+, 1159.4 [2M+Na]+.
The two diastereoisomers 7a-Rp and 7a-Sp were separated via Biotage Isolera One (cartridge SNAP-Ultra C18 12 g, F: 12 mL/min, isocratic eluent system: H2O/CH3OH 45/55 in 30 min, 150 mg sample) to obtain:
7a-Rp as Fast Eluting Isomer (76 mg)
31P NMR (202 MHz, CD3OD) δP 3.91. 1H NMR (500 MHz, CDCl3) δH 8.26 (s, 1H, H-8), 8.22 (s, 1H, H-2), 7.37–7.25 (m, 7H, Ar), 7.22–7.12 (m, 3H, Ar), 6.01 (d, J = 1.5 Hz, 1H, H-1′), 5.12 (AB q, JAB = 12.0 Hz, 2H, CH2Ph), 4.74–4.70 (m, 1H, H-2′), 4.69–4.62 (m, 1H, H-4′), 4.44–4.38 (m, 1H, H-5′), 4.28–4.21 (m, 1H, H-5′), 3.99–3.90 (m, 1H, CHCH3), 2.35–2.27 (m, 1H, H-3′), 2.09–2.02 (m, 1H, H-3′), 1.29 (d, J = 7.0 Hz, 3H, CHCH3). HPLC reversed-phase HPLC eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min, F = 1 mL/min, λ = 254 nm, showed one peak with tR 13.56 min.
7a-Sp as Slow-Eluting Isomer (61 mg)
31P NMR (202 MHz, CD3OD) δP 3.73. 1H NMR (500 MHz, CDCl3) δH 8.24 (s, 1H, H-8), 8.22 (s, 1H, H-2), 7.36–7.26 (m, 7H, Ar), 7.22–7.13 (m, 3H, Ar), 6.01 (d, J = 1.5 Hz, 1H, H-1′), 5.08 (AB q, JAB = 12.0 Hz, 2H, CH2Ph), 4.70–4.67 (m, 1H, H-2′), 4.66–4.60 (m, 1H, H-4′), 4.41–4.35 (m, 1H, H-5′), 4.26–4.19 (m, 1H, H-5′), 4.02–3.94 (m, 1H, CHCH3), 2.36–2.27 (m, 1H, H-3′), 2.08–2.01 (m, 1H, H-3′), 1.34–1.30 (m, 3H, CHCH3). HPLC reversed-phase HPLC eluting with H2O/CH3CN from 90/10 to 0/100 in 30 min, F = 1 mL/min, λ = 254 nm, tR 13.75 min.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
……
| Clinical data | |
|---|---|
| Other names | NUC-7738 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2348493-39-8 |
| PubChem CID | 166177279 |
| DrugBank | DB19148 |
| UNII | Y7BFN2M72F |
| ChEMBL | ChEMBL5277528 |
| Chemical and physical data | |
| Formula | C26H29N6O7P |
| Molar mass | 568.527 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- “Fosdesdenosine sipalabenamide”. PatSnap.
- “Fosdesdenosine Sipalabenamide”. PubChem. U.S. National Library of Medicine.
- Serpi M, Ferrari V, McGuigan C, Ghazaly E, Pepper C (December 2022). “Synthesis and Characterization of NUC-7738, an Aryloxy Phosphoramidate of 3′-Deoxyadenosine, as a Potential Anticancer Agent”. Journal of Medicinal Chemistry. 65 (23): 15789–15804. doi:10.1021/acs.jmedchem.2c01348. PMC 9743095. PMID 36417756.
….///////Fosdesdenosine sipalabenamide, antineoplastic, NUC 7738, Y7BFN2M72F














