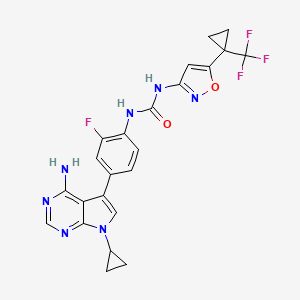
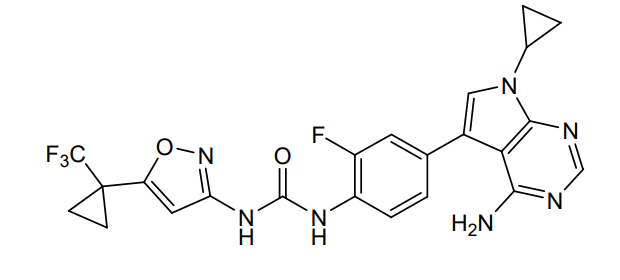
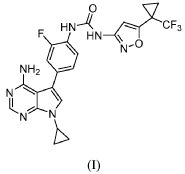
Ofirnoflast
CAS 2731294-23-6
MFC23H19F4N7O2 MW501.4 g/mol
N-[4-(4-amino-7-cyclopropyl-7H-pyrrolo[2,3-d]pyrimidin-5-yl)-2-fluorophenyl]-N’-{5-[1-
(trifluoromethyl)cyclopropyl]-1,2-oxazol-3-yl}urea
N-(4-(4-AMINO-7-CYCLOPROPYL-7H-PYRROLO(2,3-D)PYRIMIDIN-5-YL)-2-FLUOROPHENYL)-N’-(5-(1-(TRIFLUOROMETHYL)CYCLOPROPYL)-3-ISOXAZOLYL)UREA
N-(4-(4-AMINO-7-CYCLOPROPYL-7H-PYRROLO(2,3-D)PYRIMIDIN-5-YL)-2-FLUOROPHENYL)-N’-(5-(1-(TRIFLUOROMETHYL)CYCLOPROPYL)-1,2-OXAZOL-3-YL)UREA
OFIRNOLAST [USAN]
OFIRNOFLAST
UREA, N-(4-(4-AMINO-7-CYCLOPROPYL-7H-PYRROLO(2,3-D)PYRIMIDIN-5-YL)-2-FLUOROPHENYL)-N’-(5-(1-(TRIFLUOROMETHYL)CYCLOPROPYL)-3-ISOXAZOLYL)-
OFIRNOFLAST [INN]
serine/ threonine-protein kinase Nek7 inhibitor, antiinflammatory, HT-6184, HT 6184, 54PY2PBN7S
Ofirnoflast is an investigational drug, a NEK7 inhibitor, that targets and disrupts the formation of the NLRP3 inflammasome, a key driver of chronic inflammation. Developed by Halia Therapeutics, it is being explored for conditions like myelodysplastic syndromes (MDS), obesity, and Alzheimer’s disease. The drug’s unique mechanism aims to address inflammation at a root cause level, potentially offering a new approach to treating these diseases.
How it works
- Ofirnoflast is a “first-in-class” molecule that selectively inhibits the NEK7 protein.
- NEK7 is essential for the assembly of the NLRP3 inflammasome, a molecular complex that causes chronic inflammation.
- By inhibiting NEK7, ofirnoflast prevents the inflammasome from forming and promotes its disassembly.
- This approach aims to reduce inflammation without causing broad immunosuppression.
Therapeutic applications
- Myelodysplastic Syndromes (MDS): Ofirnoflast has completed a Phase 2 study for this condition and received Orphan Drug Designation from the FDA. It is being investigated for its potential to improve blood cell production by targeting the underlying inflammation.
- Obesity: An ongoing Phase 2 study is exploring ofirnoflast in combination with semaglutide to target inflammation and metabolic issues.
- Alzheimer’s Disease: Ofirnoflast is part of an early-stage program looking into its potential for this disease.
Ofirnoflast is a first-in-class, orally bioavailable NEK7 inhibitor currently undergoing Phase 2 clinical evaluation. It disrupts NLRP3 inflammasome assembly by targeting NEK7’s scaffolding function—blocking complex formation independently of NLRP3 activation status, upstream of caspase activation, pyroptosis, and inflammatory cytokine release. This mechanism offers a novel therapeutic approach for chronic inflammation. Unlike NSAIDs, corticosteroids, cytokine-neutralising biologics, and NLRP3-directed small molecules—which are frequently limited by off-target effects, immunosuppression, or incomplete efficacy—ofirnoflast provides a targeted approach with fewer anticipated liabilities
- A Ph2 Study to Evaluate the Safety, Efficacy and Tolerability of HT-6184 and Semaglutide in Obese Participants With T2DMCTID: NCT07172867Phase: Phase 2Status: Not yet recruitingDate: 2025-09-15
- HT-6184 in Subjects With MDSCTID: NCT07052006Phase: Phase 2Status: Active, not recruitingDate: 2025-07-14
- Evaluating Ability of HT-6184 to Reduce Inflammation and Pain After Third Molar ExtractionCTID: NCT06241742Phase: Phase 2Status: CompletedDate: 2025-03-30
- Study to Evaluate HT-6184 in Healthy SubjectsCTID: NCT05447546Phase: Phase 1Status: CompletedDate: 2023-08-28
SYN
https://www.tandfonline.com/doi/full/10.1080/1061186X.2025.2542856
SYN
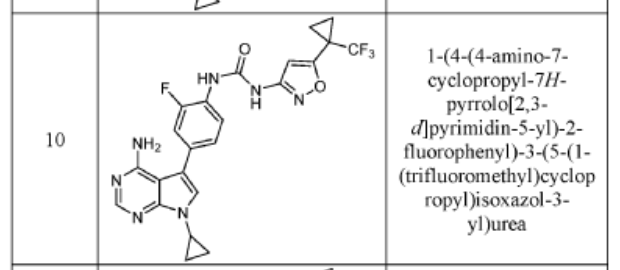
COMPD 10
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021242505&_cid=P11-MHZPDU-32878-1
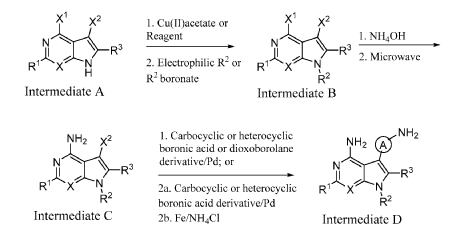
INTERMEDIATE D1
5-(4-AMINO-3-FLUOROPHENYL)-7-CYCLOPROPYL-7H-PYRROLO[2,3-D]PYRIMIDIN-4- AMINE
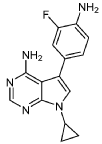
A mixture of 7-cyclopropyl-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-4-amine (C1, 0.160 g, 0.533 mmol), 2-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline (0.190 g, 0.800 mmol), and K2CO3 (0.221 g, 1.599 mmol) in 1,4-dioxane (1 mL) and water (0.3 mL) was purged with N2 for 10 min. Pd(PPh3)4 (0.062 g, 0.053 mmol) was then added and the reaction mixture was stirred at 100 °C for 12 h. Following completion of the reaction (as indicated by TLC), the mixture was filtered through a pad celite which was then rinsed with EtOAc (2 x 10 mL). The combined filtrates were concentrated under reduced pressure to yield crude material which was purified by flash chromatography (silica gel 230-400 mesh, eluting with 3% MeOH in DCM), affording
the title compound as a yellow solid (0.110 g, 73% yield).1H NMR (400 MHz, DMSO-d6) δ = 8.14 (s, 1H), 7.13 (s, 1H), 7.05-7.09 (m, 1H), 6.95-6.98 (m, 1H), 6.82-6.86 (m, 1H), 6.10 (bs, 2H), 5.22 (bs, 2H), 3.52-3.58 (m, 1H), 1.00-1.04 (m, 4H). LCMS: 284.1 [M+H].
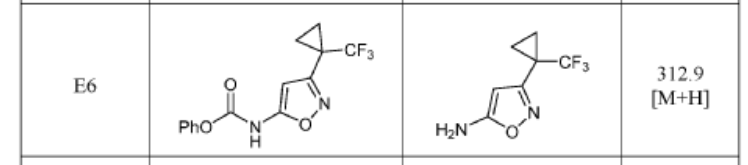
3-(1-(Trifluoromethyl)cyclopropyl)isoxazol-5-amine (precursor to E6) and 5-(1-(trifluoromethyl)cyclopropyl)isoxazol-3-amine (precursor to E7) were synthesized as reported in Synthesis 2013, 45, 171–173
EXAMPLE 5
1-(4-(4-AMINO-7-CYCLOPROPYL-7H-PYRROLO[2,3-D]PYRIMIDIN-5-YL)-2- FLUOROPHENYL)-3-(3-(1-(TRIFLUOROMETHYL)CYCLOPROPYL)ISOXAZOL-5-YL)UREA
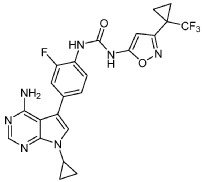
The title compound was prepared following the general procedure for urea formation (Method A), starting from 5-(4-amino-3-fluorophenyl)-7-cyclopropyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine (D1, 0.080 g, 0.282 mmol) and phenyl (3-(1-(trifluoromethyl)cyclopropyl)isoxazol-5-yl)carbamate (E6, 0.088 g, 0.282 mmol), and was obtained as a white solid (0.031 g, 22% yield).1H NMR (400 MHz, DMSO-d6) δ = 10.59 (bs, 1H), 8.84 (bs, 1H), 8.11-8.17 (m, 2H), 7.26-7.37 (m, 3H), 6.20 (s, 1H), 6.16 (bs, 2H), 3.55-3.61 (m, 1H), 1.45-1.49 (m, 2H), 1.38-1.43 (m, 2H), 1.03-1.08 (m, 4H). LCMS: 502.1 [M+H].
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2024249257&_cid=P11-MHZP9H-30149-1
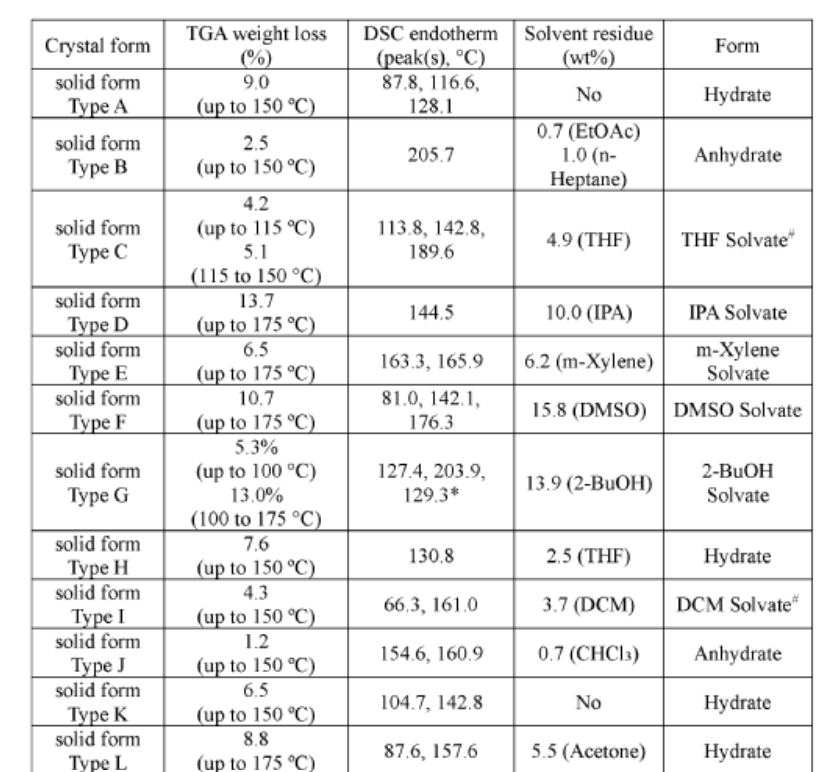
PAT
- Targeted nek7 inhibition for modulation of the nlrp3 inflammasomePublication Number: US-2023210853-A1Priority Date: 2020-05-08
- Inhibitors of NEK7 kinasePublication Number: US-11713321-B2Priority Date: 2020-05-08Grant Date: 2023-08-01
- Inhibitors of nek7 kinasePublication Number: EP-4146348-B1Priority Date: 2020-05-08Grant Date: 2024-07-03
- Inhibitors of nek7 kinasePublication Number: US-2023416259-A1Priority Date: 2020-05-08
- Inhibitors of NEK7 kinasePublication Number: US-12091413-B2Priority Date: 2020-05-08Grant Date: 2024-09-17
- Inhibitors of nek7 kinasePublication Number: TW-202208356-APriority Date: 2020-05-08
- Inhibitors of NEK7 kinasePublication Number: AU-2021280893-A1Priority Date: 2020-05-08
- Inhibitors of NEK7 kinasePublication Number: CN-115843272-APriority Date: 2020-05-08
- Inhibitors of nek7 kinasePublication Number: EP-4146348-A1Priority Date: 2020-05-08
- Inhibitors of NEK7 kinasePublication Number: KR-20230008763-APriority Date: 2020-05-08
- Polymorphs of nek 7 inhibitorsPublication Number: WO-2024249257-A1Priority Date: 2023-05-26
- Inhibitors of NEK7 kinasePublication Number: US-11161852-B1Priority Date: 2020-05-08Grant Date: 2021-11-02
- Inhibitors of nek7 kinasePublication Number: US-2021355130-A1Priority Date: 2020-05-08
- Inhibitors of nek7 kinasePublication Number: US-2022064173-A1Priority Date: 2020-05-08
- Inhibitors of nek7 kinasePublication Number: WO-2021242505-A1Priority Date: 2020-05-08



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
///////////ofirnoflast, serine/ threonine-protein kinase Nek7 inhibitor, antiinflammatory, HT-6184, HT 6184, 54PY2PBN7S














