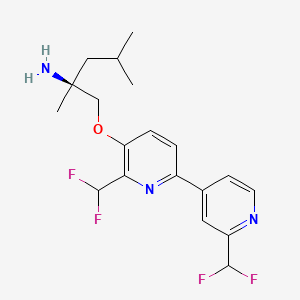

Pilavapadin
CAS1815613-42-3
MFC19H23F4N3O MW 385.4 g/mol
(2S)-1-{[2′,6-bis(difluoromethyl)[2,4′-bipyridin]-5-yl]oxy}-2,4-dimethylpentan-2-amine
(2S)-1-[[2-(difluoromethyl)-6-[2-(difluoromethyl)-4-pyridinyl]-3-pyridinyl]oxy]-2,4-dimethylpentan-2-amine
adaptor protein 2-associated kinase 1 (AAK1) inhibitor, LX9211, BMS-986176, LX 9211, BMS 986176, Phase 2, Neuropathic pain, Postherpetic neuralgia, AAK1-IN-1, 9G4RLM5X6Z
Pilavapadin (also known as LX9211 or BMS-986176) is an investigational, orally available small molecule developed by Lexicon Pharmaceuticals for the treatment of neuropathic pain, primarily diabetic peripheral neuropathic pain (DPNP).
Key Information
- Mechanism of Action: Pilavapadin is a selective inhibitor of AAK1 (AP2 associated kinase 1), a novel target identified through Lexicon’s gene science research. It is designed to inhibit the reuptake and recycling of neurotransmitters involved in pain signaling in the central nervous system without affecting opiate pathways.
- Indication: It is being investigated for the management of chronic and debilitating conditions such as diabetic peripheral neuropathic pain (DPNP), chemotherapy-induced peripheral neuropathy (CIPN), and multiple sclerosis (MS) pain.
- Development Stage: Pilavapadin has completed Phase 2 clinical trials for DPNP and is expected to advance to a Phase 3 trial.
- Status/Designation: The U.S. Food and Drug Administration (FDA) has granted Fast Track designation for the development of pilavapadin in DPNP.
Clinical Trial Results
Phase 2 studies (RELIEF-DPN-1 and PROGRESS) demonstrated that pilavapadin can provide meaningful pain reduction in adults with DPNP.
- In a post-hoc analysis of the PROGRESS study, the 10 mg dose was found to be effective, achieving a clinically meaningful, two-point reduction in average daily pain scores from baseline, with an acceptable safety and tolerability profile.
- The data has been presented at several medical meetings, including the European Association for the Study of Diabetes (EASD).
- OriginatorBristol-Myers Squibb; Lexicon Pharmaceuticals
- DeveloperLexicon Pharmaceuticals
- ClassAnalgesics; Small molecules
- Mechanism of ActionAdaptor-associated kinase 1 inhibitors
- Phase IINeuropathic pain; Postherpetic neuralgia
- 20 Jun 2025Updated efficacy data from the phase II PROGRESS trial in Neuropathic pain presented at 85th Annual Scientific Sessions of the American Diabetes Association (ADA-2025)
- 13 May 2025Lexicon Pharmaceuticals plans an End of Phase 2 meeting with FDA for Pilavapadin
- 13 May 2025Updated efficacy data from the phase II PROGRESS trial in Neuropathic pain released by Lexicon Pharmaceuticals
- A Dose-ranging Study in Patients With Diabetic Peripheral Neuropathic Pain (DPNP)CTID: NCT06203002Phase: Phase 2Status: CompletedDate: 2025-08-29
- Efficacy, Safety, and PK of LX9211 in Participants With Diabetic Peripheral Neuropathic PainCTID: NCT04455633Phase: Phase 2Status: CompletedDate: 2025-06-25
- Efficacy and Safety of LX9211 in Participants With Postherpetic NeuralgiaCTID: NCT04662281Phase: Phase 2Status: CompletedDate: 2023-11-18
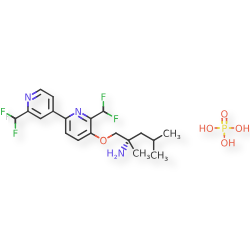
Molecular FormulaC19H23F4N3O.H3O4P
Molecular Weight483.4
CAS 2977251-24-2
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US215884039&_cid=P11-MI4ESM-19570-1
Example 123
(S)-1-((2′,6-bis(difluoromethyl)-[2,4′-bipyridin]-5-yl)oxy)-2,4-dimethylpentan-2-amine

Part A: (2-(difluoromethyl)pyridin-4-yl)boronic acid

Part B: (S)-1-((2′,6-bis(difluoromethyl)-[2,4′-bipyridin]-5-yl)oxy)-2,4-dimethylpentan-2-amine
SYN
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021216441&_cid=P11-MI4EP8-16561-2
REF
- Discovery and Optimization of Biaryl Alkyl Ethers as a Novel Class of Highly Selective, CNS-Penetrable, and Orally Active Adaptor Protein-2-Associated Kinase 1 (AAK1) Inhibitors for the Potential Treatment of Neuropathic PainPublication Name: Journal of Medicinal ChemistryPublication Date: 2022-03-09PMID: 35261239DOI: 10.1021/acs.jmedchem.1c02132
- Discovery of (S)-1-((2′,6-Bis(difluoromethyl)-[2,4′-bipyridin]-5-yl)oxy)-2,4-dimethylpentan-2-amine (BMS-986176/LX-9211): A Highly Selective, CNS Penetrable, and Orally Active Adaptor Protein-2 Associated Kinase 1 Inhibitor in Clinical Trials for the Treatment of Neuropathic PainPublication Name: Journal of Medicinal ChemistryPublication Date: 2022-03-08PMID: 35257579DOI: 10.1021/acs.jmedchem.1c02131
- Discovery, Structure–Activity Relationships, and In Vivo Evaluation of Novel Aryl Amides as Brain Penetrant Adaptor Protein 2-Associated Kinase 1 (AAK1) Inhibitors for the Treatment of Neuropathic PainPublication Name: Journal of Medicinal ChemistryPublication Date: 2021-07-16PMID: 34270254DOI: 10.1021/acs.jmedchem.1c00472
PAT
- Biaryl kinase inhibitorsPublication Number: US-2021277001-A1Priority Date: 2014-04-02
- Biaryl kinase inhibitorsPublication Number: KR-102379518-B1Priority Date: 2014-04-02Grant Date: 2022-03-25
- Biaryl kinase inhibitorsPublication Number: US-12065437-B2Priority Date: 2014-04-02Grant Date: 2024-08-20
- Biaryl kinase inhibitorsPublication Number: US-2024360131-A1Priority Date: 2014-04-02



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
////////////Pilavapadin, LX9211, BMS-986176, LX 9211, BMS 986176, Phase 2, Neuropathic pain, Postherpetic neuralgia, AAK1-IN-1, 9G4RLM5X6Z














