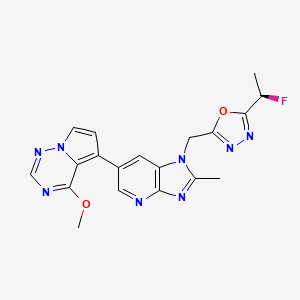
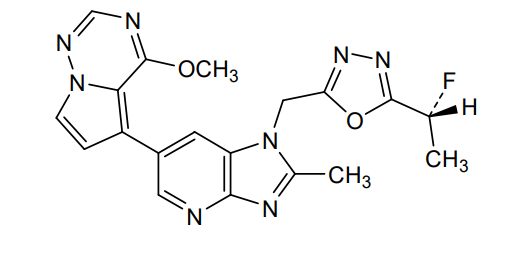
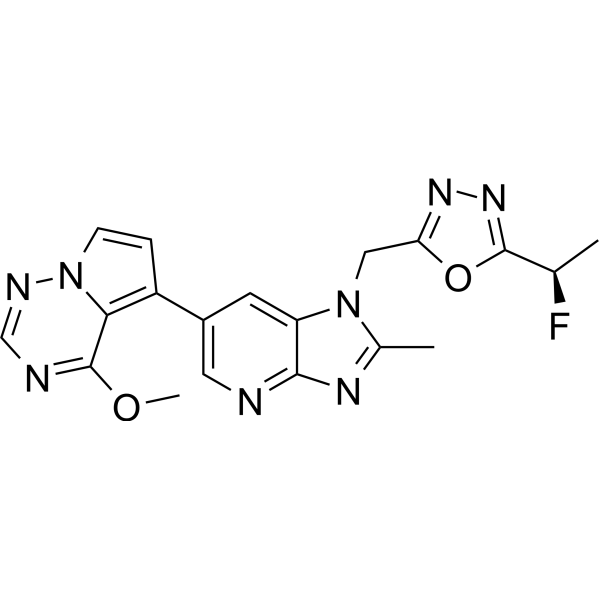
Rogocekib
CAS 2144751-78-8
MF C19H17FN8O2 MW 408.39
1-({5-[(1R)-1-fluoroethyl]-1,3,4-oxadiazol-2-yl}methyl)-6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-yl)-2-methyl1H-imidazo[4,5-b]pyridine
2-[(1R)-1-fluoroethyl]-5-[[6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-yl)-2-methylimidazo[4,5-b]pyridin-1-yl]methyl]-1,3,4-oxadiazole
dual specificity protein kinase CLK (CDC2-like kinase)inhibitor, antineoplastic, CTX 712, XE88VQP94E
Rogocekib is an orally effective CLK 2 inhibitor, with an IC50 of 1.4 nM, showing anti-tumor activity.
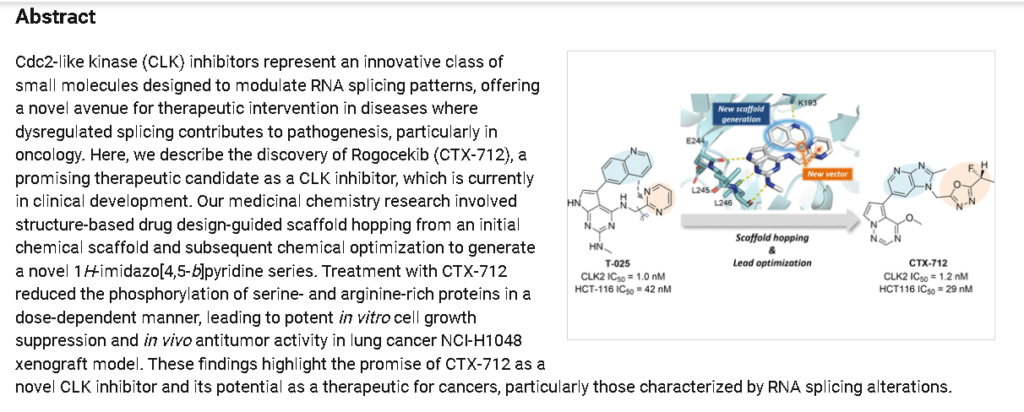
Rogocekib is an orally bioavailable inhibitor of CLK family kinases, with potential antineoplastic activity. Upon oral administration, rogocekib binds to and inhibits the activity of CLK family kinases, thereby inhibiting the phosphorylation of serine/arginine-rich (SR) domain-containing splicing factors (SFs). This modulates RNA splicing, prevents the expression of certain tumor-associated genes, and inhibits tumor cell proliferation. In many cancer cells, core spliceosome proteins, including SF3B1, U2 small nuclear ribonucleoprotein auxiliary factor 1 (U2AF1), serine/arginine-rich splicing factor 2 (SRSF2) and U2 small nuclear ribonucleoprotein auxiliary factor subunit-related protein 2 (ZRSR2), are mutated and aberrantly activated leading to a dysregulation of mRNA splicing. CLK family kinases, an evolutionarily conserved group of kinases, phosphorylates various SR proteins including SR domain-containing SFs.
SYN
https://pubs.acs.org/doi/10.1021/acsmedchemlett.5c00412
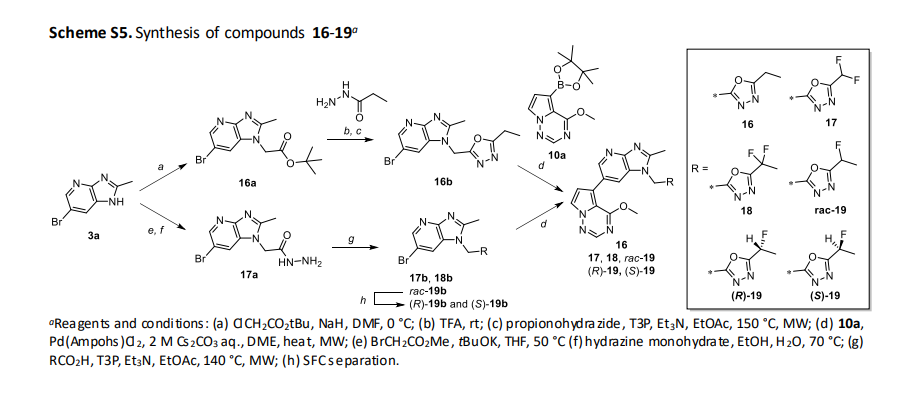
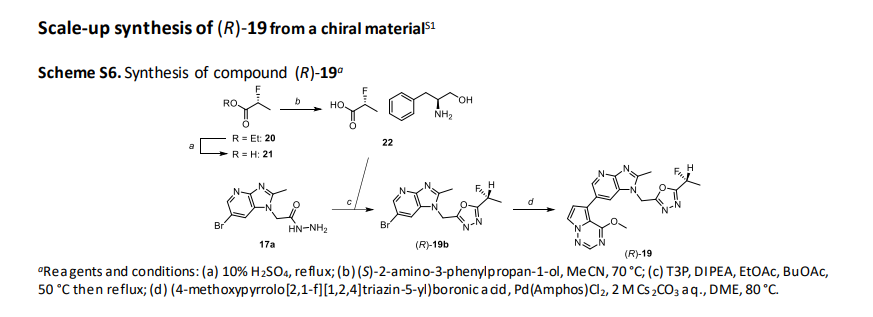
(R)-2-fluoropropanoic acid (21)
(R)-Ethyl 2-fluoropropanoate (20) (95 g, 791 mmol) was suspended in 10% sulfuric acid (950 mL), and heated and
refluxed for 3 h. After cooled, sodium chloride was added to saturate the aqueous layer, and the aqueous layer
was extracted with TBME (900 mL x4). The obtained organic layer was dried over MgSO4, and concentrated under
reduced pressure to give the title compound (124 g, 791 mmol calcd as quant., containing TBME).
1H NMR (300 MHz, DMSO-d6) δ 1.35-1.56 (3H, m), 4.91-5.21 (1H, m), 13.19 (1H, brs).
(S)-2-amino-3-phenylpropane-1-ol (R)-2-fluoropropanoate (22)
To a solution of (S)-2-amino-3-phenylpropan-1-ol (119 g, 787 mmol) in EtOH (360 mL) and MeCN (1090 mL) was
added dropwise a solution of 21 (791 mmol, theoretically calcd as quant.) in MeCN (1090 mL) at 65° C to 70° C.
The mixture was stirred at 60° C for 1 h, and further stirred at room temperature for 1 h. Precipitated crystals were
collected by filtration, and washed with MeCN (500 mL) to obtain white crystals (170 g, 699 mmol, 89%).
The obtained crystals(140 g, 575 mmol) were dissolved in EtOH (700 mL) at 60° C, and to the solution was added
MeCN (4200 mL) at 58° C to 65° C. The mixture was stirred at 60° C for 1 h. The mixture was cooled to room
temperature, and then stirred overnight at room temperature. The obtained solid was collected by filtration, and
washed with MeCN to obtain give the title compound (109 g, 448 mmol, 78%) as a white crystal.
(R)-2-((6-bromo-2-methyl-1H-imidazo[4,5-b]pyridin-1-yl)methyl)-5-(1-fluoroethyl)-1,3,4-oxadiazole ((R)-19b)
22 (109 g, 448 mmol) was dissolved in 1M HCl aq. (1500 mL) and brine (1500 mL) and extracted with TBME (1000
mL x4). The organic layer was dried over MgSO4 and concentrated in vacuo to give free salt of 22 (i.e., 21) as a
colorless oil. 50 wt% T3P in EtOAc (419 mL, 704 mmol) was added to a suspension of the above material, 17a (100
g, 351.97 mmol), and DIPEA (246 mL, 1408 mmol) in BuOAc (3000 mL) at room temperature. After being stirred at
50 °C for 30 min, 50 wt% T3P in EtOAc (210 mL, 351.97 mmol) was added to the mixture and then the mixture was
heated and refluxed for 3 h. After cooling, to the mixture was added sat NaHCO3 aq. (3000 mL), then the insoluble
material was removed by filtration. The filtrate was extracted with EtOAc (1500 mL x2). The organic layer was
separated, washed with water and brine, then passed through NH silica gel eluted with EtOAc. The residue was
concentrated in vacuo and the resulting precipitate was washed with IPE (3000 mL) to give the title compound
(57.8 g, 170 mmol, 48.3%) as an off-white solid.
1H NMR (300 MHz, DMSO-d6) δ 1.62-1.79 (3H, m), 2.62 (3H, s), 5.83-6.14 (3H, m), 8.38 (1H, d, J = 1.9 Hz), 8.45 (1H,
d, J = 1.9 Hz). MS m/z 340.0, 341.9 [M+H]+
.
1-((5-((1R)-1-fluoroethyl)-1,3,4-oxadiazol-2-yl)methyl)-6-(4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-yl)-2-methyl1H-imidazo[4,5-b]pyridine ((R)-19, CTX-712)
A mixture of (4-methoxypyrrolo[2,1-f][1,2,4]triazin-5-yl)boronic acid (79 g, 409.39 mmol), (R)-19b (100 g, 294
mmol), Pd(Amphos)Cl2 (2.00 g, 2.97 mmol), 2 M Cs2CO3 aq. (295 mL, 590 mmol) and DME (2000 mL) was stirred at
80 °C for 1 h. After cooled to 50 °C, the mixture was diluted with THF (1000 mL). The mixture was poured into
NaHCO3 aq. (1600 mL) and extracted with EtOAc (1000 mL x3). The organic layer was separated, washed with 5%
ammonia aq. (1600 mLx2) and brine (1600 mL), dried over MgSO4 and concentrated in vacuo to give a yellow solid.
To the solution of obtained solid in THF (8000 mL) and water (200 mL) was added NH silica gel (2400 g) and stirred
for 3.5 h at room temperature. The insoluble material was removed by filtration and washed with THF (15 L). The
filtrate was concentrated in vacuo to give a yellow solid. The solid was washed with TBME to give the title
compound (98 g, 240 mmol, 82 %) as a pale yellow solid. A mixture of the above material (115 g, 270 mmol) and
activated carbon (Ecosorb, 33 g) in EtOH/water = 9/1 (2200 mL) and water (1100 mL) was stirred at 55 °C for 1 h.
The insoluble material was removed by filtration, and washed EtOH (550 mL). The resultant solution was diluted
with water (1600 mL) at 55 °C and stirred at room temperature overnight. After cooled to 5 °C, the mixture was
stirred for 3 h. The solid was collected by filtration and washed with EtOH/water = 1/1 (1000 mL) to give a
colorless crystal (88 g, 207 mmol, 77% as a water adduct).
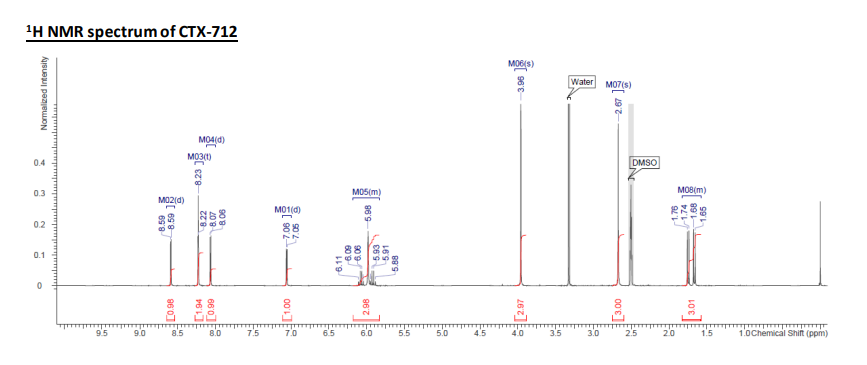
1H NMR (300 MHz, DMSO-d6) δ 1.58-1.82 (3H, m), 2.67 (3H, s), 3.96 (3H, s), 5.83-6.18 (3H, m), 7.06 (1H, d, J = 2.7
Hz), 8.06 (1H, d, J = 2.7 Hz), 8.23 (2H, t, J = 1.0 Hz), 8.59 (1H, d, J = 2.0 Hz). MS m/z 409.1 [M+H]+
.
PAT
Patent document 1:
WO 2010/016526
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010016526&_cid=P10-MIIA44-38372-1
WO 2011/096535
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023190967&_cid=P10-MII9ZT-35263-1
SYN
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=JP275206879&_cid=P10-MII9SJ-29591-1
PAT
- Condensed Heterocyclic CompoundsPublication Number: KR-102431405-B1Priority Date: 2016-04-28Grant Date: 2022-08-10
- COMPOUND, MEDICINE, AND, USE OF THE COMPOUND OR SALT THEREOFPublication Number: BR-112018072039-B1Priority Date: 2016-04-28
- Fused Heterocyclic CompoundsPublication Number: CN-109415384-BPriority Date: 2016-04-28Grant Date: 2022-01-11
- Condensed heterocyclic compoundPublication Number: EP-3450436-B1Priority Date: 2016-04-28Grant Date: 2022-07-27
- Fused heterocyclic compoundPublication Number: US-11390634-B2Priority Date: 2016-04-28Grant Date: 2022-07-19
- condensed heterocyclic compoundPublication Number: ES-2927529-T3Priority Date: 2016-04-28Grant Date: 2022-11-08
- CONDENSED HETEROCYCLIC COMPOUNDPublication Number: HR-P20221277-T1Priority Date: 2016-04-28
- Fused heterocyclic compoundPublication Number: US-10577382-B2Priority Date: 2016-04-28Grant Date: 2020-03-03
- Fused heterocyclic compoundPublication Number: US-2019106437-A1Priority Date: 2016-04-28
- Fused heterocyclic compoundPublication Number: US-2020140462-A1Priority Date: 2016-04-28
- Fused heterocyclic compoundPublication Number: US-10981934-B2Priority Date: 2016-04-28Grant Date: 2021-04-20
- Fused heterocyclic compoundPublication Number: US-2021115067-A1Priority Date: 2016-04-28
- Medicament for treatment and/or prevention of cancerPublication Number: WO-2024048541-A1Priority Date: 2022-08-30
- Biomarker for treatment of solid cancer by imidazo[4,5-b]pyridine derivativePublication Number: WO-2023190967-A1Priority Date: 2022-03-31
- Fused heterocyclic compoundPublication Number: CA-3021185-A1Priority Date: 2016-04-28
- Condensed heterocyclic compoundPublication Number: EP-3450436-A1Priority Date: 2016-04-28
- Fused heterocyclic compoundsPublication Number: JP-WO2017188374-A1Priority Date: 2016-04-28



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
- [1]. Akinori Yoda, et al. CTX-712, a Novel Clk Inhibitor Targeting Myeloid Neoplasms with SRSF2 Mutation. Blood. (2021) 205–206[2]. Zhen Qin, et al. Development of Cdc2-like Kinase 2 Inhibitors: Achievements and Future Directions. J Med Chem. 2021 Sep 23;64(18):13191-13211. [Content Brief]
///////rogocekib, CTX 712, XE88VQP94E














