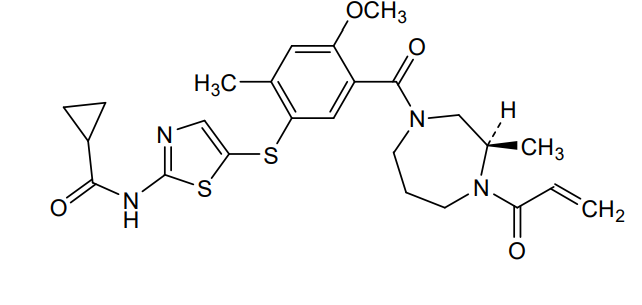
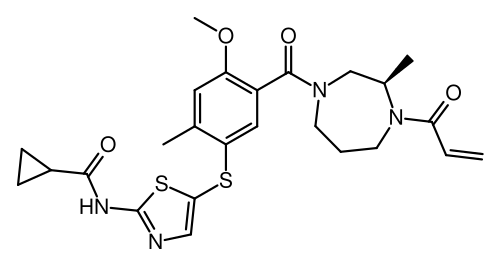
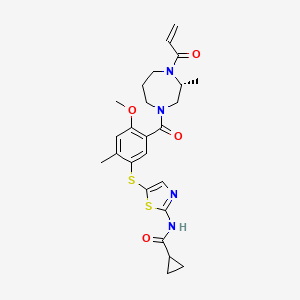
Soquelitinib
CAS 2226636-04-8
MF C25H30N4O4S2, 514.7 g/mol
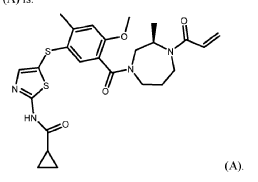
N-[5-({4-methoxy-2-methyl-5-[(3R)-3-methyl-4-(prop-2-enoyl)-1,4-diazepane-1-carbonyl]phenyl}sulfanyl)-1,3-thiazol-2-yl]cyclopropane-1-carboxamide
tyrosine kinase inhibitor, antineoplastic, CPI818, CPI-000818, CPI596, CP I818, CPI 000818, CP I596, 6I5H17AN3I,
Soquelitinib (CPI-818) is an experimental drug which acts as a selective inhibitor of the enzyme interleukin-2-inducible T-cell kinase (ITK). It is in clinical trials for the treatment of T-cell lymphoma.[1][2]
Soquelitinib is an orally available, small-molecule, irreversible inhibitor of interleukin-2 inducible T-cell kinase (ITK) with potential immunomodulatory and antineoplastic activities. Upon oral administration, soquelitinib selectively and covalently binds to the cysteine residue at position 442 (CYS-442) of ITK, thereby disrupting ITK-mediated signal transduction, while sparing tyrosine-protein kinase TXK (resting lymphocyte kinase, RLK) activity. This may abrogate T-cell receptor (TCR) signaling through ITK and inhibit TCR-induced proliferation of malignant T-cells. Additionally, inhibiting ITK activation may prevent the upregulation of GATA-3, a transcription factor that drives T-helper 2 (Th2) cell differentiation and is overexpressed in certain T-cell lymphomas. Thus, selective inhibition of ITK may inhibit Th2 responses without affecting T-helper 1 (Th1)-dependent immunity. ITK, a member of the Tec family of non-receptor protein tyrosine kinases plays a significant role in the T-cell development, differentiation and production of pro-inflammatory cytokines.
- Safety, Tolerability, and Preliminary Efficacy of Soquelitinib in Participants With Moderate to Severe ADCTID: NCT06345404Phase: Phase 1Status: RecruitingDate: 2025-07-22
- Study of the ITK Inhibitor Soquelitinib to Reduce Lymphoproliferation and Improve Cytopenias in Autoimmune Lymphoproliferative Syndrome (ALPS)-FAS PatientsCTID: NCT06730126Phase: Phase 2Status: RecruitingDate: 2025-05-31
- Soquelitinib vs Standard of Care in Participants With Relapsed/Refractory Peripheral T-cell Lymphoma Not Otherwise Specified, Follicular Helper T-cell Lymphomas, or Systemic Anaplastic Large-cell LymphomaCTID: NCT06561048Phase: Phase 3Status: RecruitingDate: 2025-04-17
- A Dose Escalation Study Evaluating CPI-818 in Relapsed/Refractory T-Cell LymphomaCTID: NCT03952078Phase: Phase 1Status: Active, not recruitingDate: 2025-04-16
Syn
- US11008314,
- https://patentscope.wipo.int/search/en/detail.jsf?docId=US278926237&_cid=P10-MISM56-82578
- SIMILAR
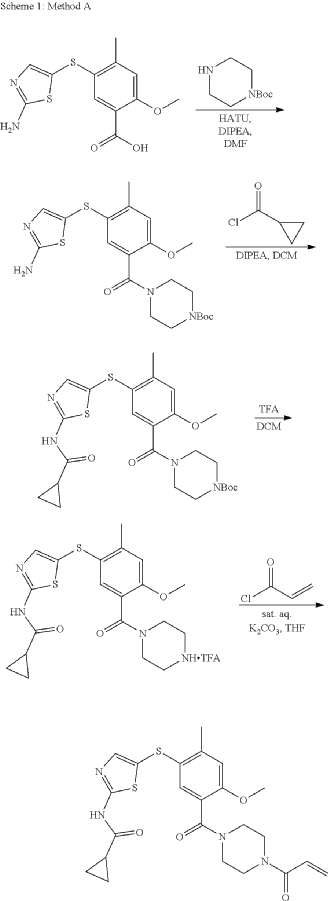

Syn
- WO2018089261 COMPD 44
- https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018089261&_cid=P10-MISM0C-78029-1
SYN
Embodiment B23. A method for an Th2/ITK-mediated disease in a patient in need thereof, the method comprising administering to the patient about 250 mg to about 1,000 mg per day of a compound of Formula (A) or a pharmaceutically acceptable salt thereof, wherein the compound of Formula (A) is:
REF
https://www.nature.com/articles/s44386-024-00002-1
Pat
- Compounds and methods for modulating interleukin-2-inducible t-cell kinasePublication Number: US-2022363676-A1Priority Date: 2016-11-03
- Compounds and methods for modulating Interleukin-2-inducible T-cell kinasePublication Number: US-11897874-B2Priority Date: 2016-11-03Grant Date: 2024-02-13
- Itk inhibitors for increasing th1 cell activityPublication Number: WO-2023196278-A1Priority Date: 2022-04-05
- Compounds and methods for modulating interleukin-2-inducible t-cell kinasePublication Number: US-2019375743-A1Priority Date: 2016-11-03
- Compounds and methods for modulating interleukin-2-inducible t-cell kinasePublication Number: WO-2018089261-A2Priority Date: 2016-11-03
- Compounds and methods for modulating interleukin-2-inducible t-cell kinasePublication Number: US-11008314-B2Priority Date: 2016-11-03Grant Date: 2021-05-18
- Compounds and methods for modulating interleukin-2-inducible t-cell kinasePublication Number: EP-3534899-B1Priority Date: 2016-11-03Grant Date: 2022-06-01



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Khodadoust MS, Feldman TA, Yoon DH, Yannakou CK, Radeski D, Kim YH, et al. (November 2020). “Cpi-818, an oral interleukin-2-inducible T-cell kinase inhibitor, is well-tolerated and active in patients with T-cell lymphoma”. Blood. 136: 19–20. doi:10.1182/blood-2020-137782.
- Hsu LY, Rosenbaum JT, Verner E, Jones WB, Hill CM, Janc JW, et al. (December 2024). “Synthesis and characterization of soquelitinib a selective ITK inhibitor that modulates tumor immunity”. npj Drug Discovery. 1 (1) 2: 1–4. doi:10.1038/s44386-024-00002-1.
| Identifiers | |
|---|---|
| IUPAC name | |
| CAS Number | 2226636-04-8 |
| PubChem CID | 134517711 |
| DrugBank | DB18749 |
| ChemSpider | 129629996 |
| UNII | 6I5H17AN3I |
| KEGG | D12762 |
| Chemical and physical data | |
| Formula | C25H30N4O4S2 |
| Molar mass | 514.66 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
//////////////Soquelitinib, tyrosine kinase inhibitor, antineoplastic, CPI818, CPI-000818, CPI596, CP I818, CPI 000818, CP I596, 6I5H17AN3I,














