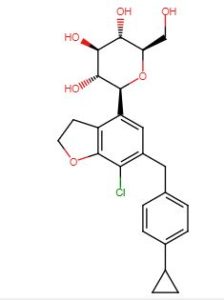

Enavogliflozin, DWP-16001
(2S,3R,4R,5S,6R)-2-(7-chloro-6-(4-cyclopropylbenzyl)-2,3-dihydrobenzofuran-4-yl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
CAS: 1415472-28-4
Chemical Formula: C24H27ClO6
Molecular Weight: 446.92
Elemental Analysis: C, 64.50; H, 6.09; Cl, 7.93; O, 21.48
Green Cross Corp INNOVATOR
Daewoong Pharmaceutical Co Ltd
Enavogliflozin is an antidiabetic (hypoglycemic).
Daewoong is investigating DWJ-304 , a sodium/glucose cotransporter 2 (SGLT-2) inhibitor, for treating type 2 diabetes. By February 2017, preclinical development was underway. Daewoong is developing DWP-16001 , presumed to be enavogliflozin, a SGLT-2 inhibitor, for treating type 2 diabetes. In September 2019, launch was expected in 2023.
Enavogliflozin (DWP16001/GCC5694A) is a selective SLC5A2 and SGLT2 inhibitor developed to treat diabetes and obesity.[1][2][3][4][5] It was developed by GC Pharma[6][7] and Daewoong Pharmaceutical.[7]
Enavogliflozin has been approved for clinical use in South Korea,[7] and Ecuador,[8] and applied for approval in Brazil, Mexico, Peru, and Colombia.[8]
PATENT
WO2012165914
DWP-16001 expire in EU states until June 2032 and US in November 2033.
PATENT
US 2014274918
PATENT
US2019169174
Paragraph 0305; 0340; 0347
H NMR (400 MHz, CD3OD) δ 7.02 (d, J=8.0 Hz, 2H), 6.92 (d, J=8.0 Hz, 2H), 6.81 (s, 1H), 4.59 (t, J=8.8 Hz, 2H), 4.11 (d, J=9.2 Hz, 1H), 3.96 (ABq, ΔvAB=19.0 Hz, JAB=15.2 Hz, 2H), 3.87-3.84 (m, 1H), 3.67-3.63 (m, 1H), 3.47-3.37 (m, 3H), 3.35-3.33 (m, 3H), 1.85-1.79 (m, 1H), 0.91-0.86 (m, 2H), 0.61-0.57 (m, 2H)
PATENT
WO2017217792 , claiming process for preparing diphenylmethane derivative.
1H NMR(400 MHz, CD 3OD) δ 7.02(d, J = 8.0 Hz, 2H), 6.92(d, J = 8.0 Hz, 2H), 6.81(s, 1H), 4.59(t, J = 8.8 Hz, 2H), 4.11(d, J = 9.2 Hz, 1H), 3.96(ABq, Δv AB = 19.0 Hz, J AB = 15.2 Hz, 2H), 3.87-3.84(m, 1H), 3.67-3.63(m, 1H), 3.47-3.37(m, 3H), 3.35-3.33(m, 3H), 1.85-1.79(m, 1H), 0.91-0.86(m, 2H), 0.61-0.57(m, 2H); [M+Na] + 469.
PATENT
WO-2020036382
The present invention relates to a method for producing an intermediate useful for the synthesis of a diphenylmethane derivative that can be used as a SGLT inhibitor. A method for synthesizing a compound of formula 7 according to the present invention has solved the problem of an existing synthesis process which requires an additional process due to the synthesis of Grignard reagent and the management of a related substance. In addition, the process can be simplified by minimizing the formation of the related substance and eliminating the need for reprocessing of reaction products, thereby becoming capable of maximizing a yield of a diphenylmethane derivative.
Process for preparing intermediates of SGLT inhibitor and their use for the synthesis of diphenyl-methane derivative, which can be used as SGLT inhibitors.
REFERENCES
1: Markiewicz M, Jungnickel C, Stolte S, Białk-Bielińska A, Kumirska J, Mrozik W. Ultimate biodegradability and ecotoxicity of orally administered antidiabetic drugs. J Hazard Mater. 2017 Jul 5;333:154-161. doi: 10.1016/j.jhazmat.2017.03.030. Epub 2017 Mar 16. PubMed PMID: 28349868.
2: Holt RI. Trials of new anti-diabetes agents. Diabet Med. 2017 Feb;34(2):147. doi: 10.1111/dme.13306. PubMed PMID: 28090726.
References
- Hwang, Jun Gi; Lee, SeungHwan; Huh, Wan; Han, Jumi; Oh, Jaeseong; Jang, In-Jin; Yu, Kyung-Sang (September 2022). “Dose-dependent glucosuria of DWP16001, a novel selective sodium–glucose cotransporter-2 inhibitor, in healthy subjects”. British Journal of Clinical Pharmacology. 88 (9): 4100–4110. doi:10.1111/bcp.15348. PMID 35395697.
- Kim, Ju-Hyun; Kim, Dong Kyun; Choi, Won-Gu; Ji, Hye-Young; Choi, Ji-Soo; Song, Im-Sook; Lee, Sangkyu; Lee, Hye Suk (11 September 2020). “In Vitro Metabolism of DWP16001, a Novel Sodium-Glucose Cotransporter 2 Inhibitor, in Human and Animal Hepatocytes”. Pharmaceutics. 12 (9): 865. doi:10.3390/pharmaceutics12090865. PMC 7558535. PMID 32932946.
- Kim, Byungwook; Huh, Ki Young; Hwang, Jun Gi; Nah, JaeJin; Huh, Wan; Cho, Jae Min; Jang, In-Jin; Yu, Kyung-Sang; Kim, Yun; Lee, SeungHwan (April 2023). “Pharmacokinetic and pharmacodynamic interaction between DWP16001, an sodium–glucose cotransporter 2 inhibitor and metformin in healthy subjects”. British Journal of Clinical Pharmacology. 89 (4): 1462–1470. doi:10.1111/bcp.15613. PMID 36422809. S2CID 253838705.
- Rhee, Beomseok; Mahbubur, Rahman Md; Jin, Changfan; Choi, Ji-Soo; Lim, Hyun-Woo; Huh, Wan; Park, Joon Seok; Han, Jumi; Kim, Sokho; Lee, Youngwon; Park, Jinho (December 2022). “Evaluation of safety and anti-obesity effects of DWP16001 in naturally obese dogs”. BMC Veterinary Research. 18 (1): 237. doi:10.1186/s12917-022-03324-2. PMC 9214997. PMID 35733159.
- Yoon, Sukyong; Park, Min Soo; Jin, Byung Hak; Shin, Hyobin; Na, Jaejin; Huh, Wan; Kim, Choon Ok (3 July 2023). “Pharmacokinetic and pharmacodynamic interaction of DWP16001, a sodium-glucose cotransporter-2 inhibitor, with phentermine in healthy subjects”. Expert Opinion on Drug Metabolism & Toxicology. 19 (7): 479–485. doi:10.1080/17425255.2023.2249397. PMID 37593838. S2CID 265846294.
- Kong, Young Kyu; Song, Kwang-Seop; Jung, Myung Eun; Kang, Misuk; Kim, Hyeon Jung; Kim, Min Ju (2022-01-15). “Discovery of GCC5694A: A potent and selective sodium glucose co-transporter 2 inhibitor for the treatment of type 2 diabetes”. Bioorganic & Medicinal Chemistry Letters. 56: 128466. doi:10.1016/j.bmcl.2021.128466. ISSN 0960-894X.
- Kim, Min-Soo; Song, Yoo-Kyung; Choi, Ji-Soo; Ji, Hye Young; Yang, Eunsuk; Park, Joon Seok; Kim, Hyung Sik; Kim, Min-Joo; Cho, In-Kyung; Chung, Suk-Jae; Chae, Yoon-Jee; Lee, Kyeong-Ryoon (2023-03-14). “Physiologically Based Pharmacokinetic Modelling to Predict Pharmacokinetics of Enavogliflozin, a Sodium-Dependent Glucose Transporter 2 Inhibitor, in Humans”. Pharmaceutics. 15 (3): 942. doi:10.3390/pharmaceutics15030942. ISSN 1999-4923. PMC 10058973. PMID 36986803.
- “Daewoong Pharmaceutical’s diabetes drug wins approval in Ecuador – 매일경제 영문뉴스 펄스(Pulse)”. Pulse (in Korean). 2024. Retrieved 2025-04-04.
 |
|
| Clinical data | |
|---|---|
| Other names | DWP16001 |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H27ClO6 |
| Molar mass | 446.92 g·mol−1 |
| 3D model (JSmol) | |
/////// DWJ-304, Daewoong Pharmaceutical, DWP-16001, SGLT-2 inhibitor, type 2 diabetes, KOREA, Enavogliflozin, GCC 5694A
ClC1=C2C(CCO2)=C([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)C=C1CC4=CC=C(C5CC5)C=C4
SYN
Synthesis 2024, 56, 906–943
Enavogliflozin (17) (DWP-16001) was developed by Green Cross Corp. and Daewoong Pharmaceuticals Co. Ltd.with the intention of addressing type 2 diabetes. Its chemical name is (2S,3R,4R,5S,6R)-2-(7-chloro-6-(4-cyclopropylbenzyl)-2,3-dihydrobenzofuran-4-yl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol. In clinical trials, this medication exhibited remarkable efficacy as both an antidiabetic agent and an SGLT inhibitor. The initial synthetic pathway for producing envogliflozin (17), in addition to other C-aryl-glycoside-type derivatives, was documented in the United States, specifically through patent application
number US9034921B2.75 Enavogliflozin (17) belongs to the category of C-aryl glycoside derivatives and its synthesis encompasses 19 steps, ultimately achieving an overall yield of 12%.
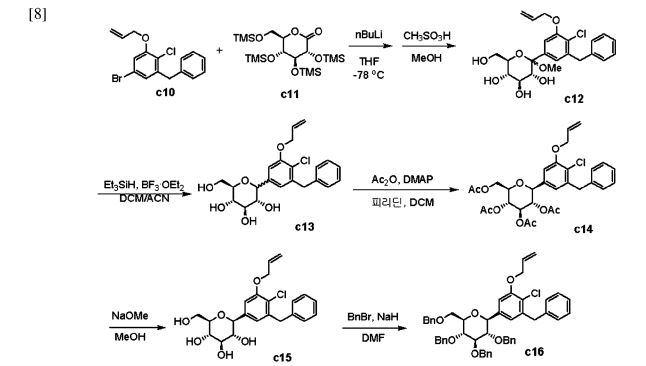
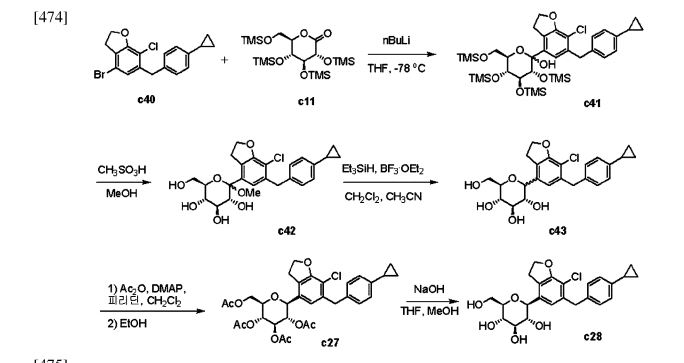
The process starts with the preparation of aglycone key intermediate 290 (Scheme 50), and involves a series chemical transformations starting from the commercially available 3-methoxy-2-nitrobenzoic acid (275). Following the successful synthesis of the aglycone intermediate 290, the process was advanced by employing n-BuLi for lithium–halogen exchange on 290 and subsequent addition of lithiated 290 to O-silyl-protected compound 22 at –78 °C (Scheme51). This sequence yielded the TMS-protected lactol inter
mediate 291 in quantitative yield. By subjecting this intermediate to treatment with MsOH/MeOH, the desired product 292 was obtained, achieving a 2-step overall yield of 88%. During these reactions, the O-silyl groups of the C-glucoside 292 were cleaved. Furthermore, the reduction of intermediate 292 was executed in the presence of triethylsilane and boron trifluoride–diethyl etherate complex, lead
ing to the formation of desmethoxy intermediate 293 in 100% yield. Subsequent acetylation of the four hydroxy groups was performed using acetic anhydride and a catalytic quantity of DMAP, producing the tetra-acetyl intermediate 294 in a yield of 59%. Ultimately, removal of the acetyl groups was achieved in the presence of NaOH, culminating in the generation of the final product, enovagliflozin (17),
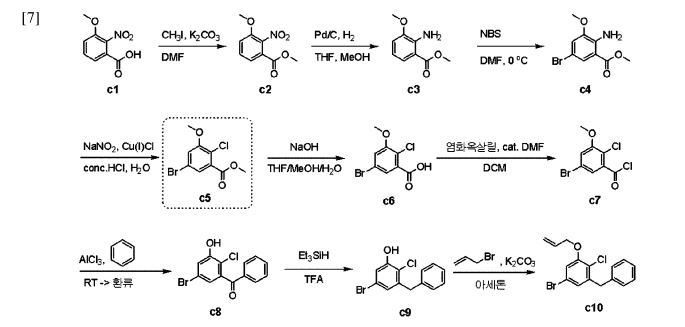
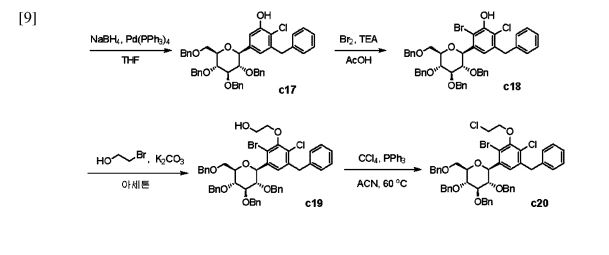
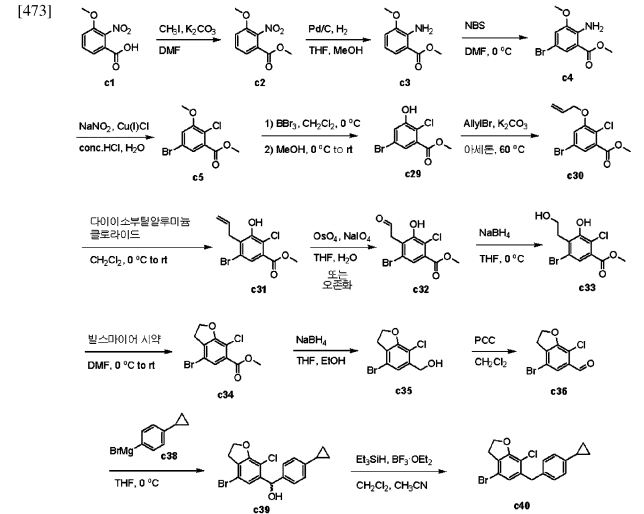
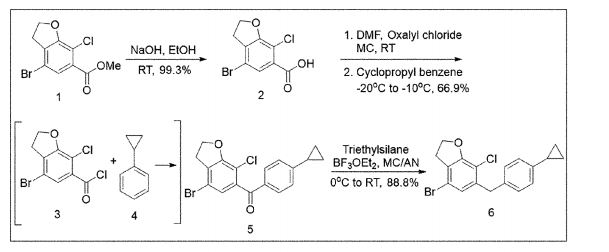
with a 100% yield (Scheme 51). This synthetic procedure is plagued by significant limitations, including an extended route to obtain the aglycone intermediate 290, the application of protection and deprotection chemistry, and the necessity of cryogenic conditions to obtain the lactol intermediate 291. According to the reaction sequences given in Schemes 50 and 51, the overall yield of the final compound is calculated to be 12% via a total of 19 steps. In another approach, enavogliflozin (17) was synthesized in 27 steps with an overall yield of 0.5% (Schemes 52and 53).76 Initially, the synthesis of O-allyl aglycone intermediate 299 was achieved in nine steps starting from 3 methoxy-2-nitrobenzoic acid (275) (see Scheme 51). Methylation of compound 275 using methyl iodide and potassium carbonate in DMF afforded methyl ester 276 in 98% yield. Reduction of the NO2 group of 276 was carried out using Pd/C to afford aryl amine 277 in excellent yield. Next, bromination of 277 was facilitated by using NBS in DMF and
ethyl acetate to afford the brominated compound 278 in 86% yield. Chlorination was then carried out on intermediate 278 under diazotization reaction conditions to afford 279. The ester group of 279 was hydrolyzed under basic conditions to afford the aryl carboxylic acid 295. Subsequently, the preparation of acid chloride 296 from 295 was achieved using oxalyl chloride and a catalytic amount of DMF, which was coupled with benzene under Friedel Crafts acylation conditions to give the aryl benzophenone
intermediate 297. Reduction of the keto group of 297 was achieved by using triethylsilane and TFA/TfOH. Finally, the O-allyl aglycone intermediate 299 was obtained, when in termediate 298 was subjected to O-allylation using allylbromide and potassium carbonate in acetone. Next, the O-allyl aglycone intermediate 299 was subjected to a Br/Li exchange reaction using n-BuLi and addition of the obtained lithiated compound was carried out on gluconolactone 22 to afford the lactol intermediate 300 in quantitative yield (Scheme 53). The hydroxy group of 300 was methylated using methanesulfonic acid in methanol to
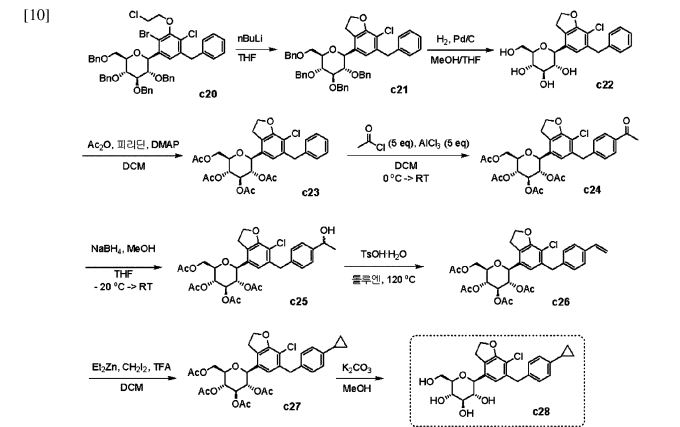
give 301 in 88% yield over two steps from 299. Demethoxylation and TMS cleavage was carried out on 301 using triethylsilane and BF3·Et2O to furnish intermediate 302. This hydroxy intermediate was protected using acetic anhydride and DMAP to afford the acetylated compound 303, deprotection of which with sodium methoxide gave product 304 in 100% yield. Benzylation of 304 was carried out using BnBr and NaH to give tetra-O-benzylated compound 305. Next, the O-allyl group of 305 was reduced to give alcohol 306 in 95% yield. Bromination followed by O-alkylation of the intermediate 306 then furnished compound 308. The hydroxy group of 308 was replaced by a chlorine atom under treatment with CCl4 and PPh3 to afford 309. Using n-BuLi, intramolecular cyclization was carried out to give com
pound 310 in 69% yield and subsequent debenzylation by treatment with Pd/C and H2 afforded compound 311. Again, acetylation of the free hydroxy groups of 311 was achieved using acetic anhydride and DMAP to give the O-acetylated intermediate 312. A Friedel–Crafts reaction on the aryl moiety of intermediate 312 gave acylated product 313 in 93% yield. The keto group of 313 was reduced using sodium borohydride in methanol to give the 314, which was further reduced under acidic conditions to give the alkene intermediate 315. The Simmons–Smith cyclopropanation was achieved on the alkene intermediate 315 to give compound 294 in 60% yield. Finally, the acetyl groups were removed
from the sugar moiety of 294 to give enavogliflozin (17) in 47% yield. This synthetic route also contains major disadvantages in terms of the use of protection/deprotection strategies, a lengthy linear process and employs several harmful reagents.
(75) Choi, S.; Song, K. S.; Lee, S. H.; Kim, M. J.; Seo H. J.; Park, E.-J.; Kong, Y.; Park, S. O.; Kang, H.; Jung, M. E.; Lee, K.; Kim, H. J.; Lee, J. S.; Lee, M. W.; Kim, M.-S.; Hong, D. H.; Kang, M. US9034921B2, 2015.
(76) Yoon, H.-K.; Park, S.-H.; Yoon, J.-S.; Choi, S.; Seo, H. J.; Park, E.-J.; Kong, Y.; Song, K.-S.; Kim, M. J.; Park S. O. WO2017217792A1, 2017.



,