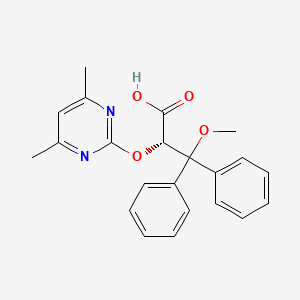
Ambrisentan
BSF-208075; LU-208075
(+)-(2S)-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropanoic acid
- Molecular FormulaC22H22N2O4
- Average mass378.421 Da
Ambrisentan (U.S. trade name Letairis; E.U. trade name Volibris; India trade name Pulmonext by MSN labs) is a drug indicated for use in the treatment of pulmonary hypertension.
The peptide endothelin constricts muscles in blood vessels, increasing blood pressure. Ambrisentan, which relaxes those muscles, is an endothelin receptor antagonist, and is selective for the type A endothelin receptor (ETA).[1] Ambrisentan significantly improved exercise capacity (6-minute walk distance) compared with placebo in two double-blind, multicenter trials (ARIES-1 and ARIES-2).[2]
Ambrisentan was approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency, and designated an orphan drug, for the treatment of pulmonary hypertension.[3][4][5][6][7]
Ambrisentan is an endothelin receptor antagonist used in the therapy of pulmonary arterial hypertension (PAH). Ambrisentan has been associated with a low rate of serum enzyme elevations during therapy, but has yet to be implicated in cases of clinically apparent acute liver injury.
Ambrisentan was first approved by the U.S. Food and Drug Administration (FDA) on Jun 15, 2007, then approved by the European Medicines Agency (EMA) on Apr 21, 2008 and approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) on Jul 23, 2010. In 2000, Abbott, originator of ambrisentan, granted Myogen (acquired by Gilead in 2006) a license to the compound for the treatment of PAH. In 2006, GlaxoSmithKline obtained worldwide rights to market the compound for PAH worldwide, with the exception of the U.S. It is marketed as Letairis® by Gilead in US.
Ambrisentan is an endothelin receptor antagonist, and is selective for the type A endothelin receptor (ETA). It is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability and delay clinical worsening. Studies establishing effectiveness included predominantly patients with WHO Functional Class II-III symptoms and etiologies of idiopathic or heritable PAH (64%) or PAH associated with connective tissue diseases (32%).
Letairis® is available as film-coated tablet for oral use, containing 5 or 10 mg of free Ambrisentan. The recommended starting dose is 5 mg once daily with or without food, and increase the dose to 10 mg once daily if 5 mg is tolerated.
Recent Developments and Publications
| Last Updated 9/2/2015 | |
|---|---|
| 8/15/2015Reprod. Toxicol. | Endothelin receptor activation mediates strong pulmonary vasoconstriction and positive inotropic effect on the heart. These physiologic effects are vital for the development of the fetal cardiopulmonary system. As such, endothelin receptor antagonists such as Ambrisentan are teratogenic.[8] |
| 8/27/2015NEJM | Ambrisentan when used in combination therapy with Tadalafil was found to be more efficacious in treating treatment naive patients with WHO class II or III Pulmonary Arterial Hypertension than monotherapy using either drug.[9] |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2007-06-15 | Marketing approval | Letairis | Pulmonary arterial hypertension | Tablet, Film coated | 5 mg/10 mg | Gilead | Priority; Orphan |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2008-04-21 | Marketing approval | Volibris | Pulmonary arterial hypertension | Tablet, Film coated | 5 mg/10 mg | GlaxoSmithKline | Orphan |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2010-07-23 | Marketing approval | Volibris | Pulmonary arterial hypertension | Tablet, Film coated | 2.5 mg | GlaxoSmithKline |
| Approval Date | Approval Type | Trade Name | Indication | Dosage Form | Strength | Company | Review Classification |
|---|---|---|---|---|---|---|---|
| 2010-10-19 | Marketing approval | 凡瑞克/Volibris | Pulmonary arterial hypertension | Tablet | 5 mg | GlaxoSmithKline | |
| 2010-10-19 | Marketing approval | 凡瑞克/Volibris | Pulmonary arterial hypertension | Tablet | 10 mg | GlaxoSmithKline |
Clinical uses
Ambrisentan is indicated for the treatment of pulmonary arterial hypertension (WHO Group 1) in patients with WHO class II or III symptoms to improve exercise capacity and delay clinical worsening.

Birth defects
Endothelin receptor activation mediates strong pulmonary vasoconstriction and positive inotropic effect on the heart. These physiologic effects are vital for the development of the fetal cardiopulmonary system. In addition to this, endothelin receptors are also known to play a role in neural crest cell migration, growth, and differentiation. As such, endothelin receptor antagonists such as Ambrisentan are known to be teratogenic.
Ambrisentan has a high risk of liver damage, and of birth defects if a woman becomes pregnant while taking it. In the U.S., doctors who prescribe it, and patients who take it, must enroll in a special program, the LETAIRIS Education and Access Program (LEAP), to learn about those risks. Ambrisentan is available only through specialty pharmacies.
External links
- Letairis website run by Gilead Sciences
- Prescribing information
- Information on the LETAIRIS Education and Access Program (LEAP)
PATENT
WO9611914A1 / US7109205B2.
WO2010070658A2 / US2011263854A1.
WO2011004402A2 / US2012184573A1.
WO2013030410A2 / US2014011992A1.
CN103709106A.
CN103420811A.
PATENT
https://patents.google.com/patent/WO2012167406A1/en
Ambrisentan and darusentan first reported in U. Med. Chem. 1996, 39, 2123-2128), as a selective antagonist of endothelin receptor A, followed by their pharmacological properties have been studied further, published in J. Med. Chem. 1996, 39, 2123-2128), US patent US 5932730, WO 2009/017777 A2 in. The formula (I), when R is methyl, Chinese name is (+) – ambrisentan, Chinese chemical name is (+) – (2S) -2 – [(4, 6- dimethyl-pyrimidine 2-yl) – oxy] -3-methoxy-3,3-diphenyl-propionic acid; English name is (+) – ambrisentan, English name: (S) -2- (4,6-dimethylpyrimidin -2-yloxy) -3-methoxy-3,3-diphenylpropanoic acid; when R is methoxy, Chinese as (+) – darusentan, Chinese chemical name is (+) – (2S) -2- [ (4,6-dimethoxypyrimidin-2-yl) – oxy] -3-methoxy-3,3-diphenyl-propionic acid; English name is (+ darusentan, English name: (S) – . 2- (4,6-dimethoxypyrimidin-2-yloxy) -3-methoxy-3,3-diphenylpropanoic acid ambrisentan now been approved by the FDA in the United States, the trade name Letairis, for the oral treatment of pulmonary hypertension; up Lu bosentan new drugs may be resistant hypertension (Resistant hypertension) of.
Existing ambrisentan or darusentan synthetic techniques include benzophenone Darzens reaction of an epoxy compound and a racemic methyl chloroacetate, the racemic epoxide opening catalyst in a solution of boron trifluoride diethyl ether ring to give the chiral alcohol latent after substitution reaction and then after hydrolysis reaction ambrisentan or darusentan. Existing obtained optically pure (+) – ambrisentan or (+) – darusentan methods rely mainly on resolution techniques. For example, the split is by a latent chiral alcohol or R- L- proline methyl phenethylamine, see WO 2010/070658 A2, WO 2011/004402 A2. It is well known as chiral utilization of raw materials is not high, resulting in increased costs, limiting industrial-scale applications.
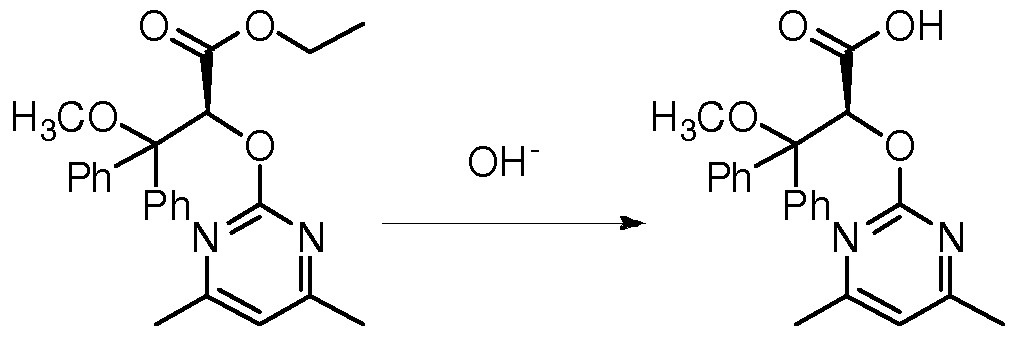
Example 1, (+) – ambrisentan ((2S) -2 – [(4,6- dimethyl-pyrimidin-2-yl) – oxy] -3-methoxy-3,3-diphenyl propionic acid) of
Preparation of 3,3-diphenyl-2,3-epoxy-propionate (1) (2S)
As indicated above Formula Scheme, wherein, Ph is phenyl; Ac is acetyl;
To a 50 L reactor equipped with a mechanical stirrer was added 3.0 L of acetonitrile was dissolved in 3,3-diphenyl acrylate (0.536 mol, 135.0 g), was dissolved in 1.5 L of acetonitrile to give a concentration of 0.12 M 4 M ethylenediamine of formula (IV) shown fructose derived chiral ketones and tetra-n-butylammonium hydrogen sulfate (36 mmol, 12.2 g), was then added containing 3.0 L Ι χ ΙΟ “an aqueous solution of disodium ; cooling liquid into the reaction vessel dissection, the kettle temperature adjusted to -5 ° C- + 5 ° C; was added in batches with stirring pulverized with the pulverizer medicine through a 1.85 kg potassium hydrogen sulfate complex salt mixture (Oxone®), and 0.78 kg NaHCO 3 (9.29mol), and takes about 4.5 hours complete addition of the above mixture, after the addition the reaction mixture was continued stirring the reaction under this condition (in the system, 3,3- diphenyl acrylate, over a potassium bisulfate salts and complexes of formula molar ratio of fructose derived chiral ketone (IV) is shown in h 5: 0.34), and the timing detection reactions by gas chromatography; the end of the reaction after 5 hours , 5.0 L of water was added to dilute the reaction solution, and extracted with 5.0 L of ethyl acetate; the aqueous phase was added 2.5 L of acetic Extracted with ethyl; organic phases were combined and concentrated to remove the solvent to give homogeneous Qing 162.56 g (2S) -3,3- diphenyl-2,3-epoxy-propionate, crude yield greater than 99%, No purification processing the next reaction, nuclear magnetic conversion was 92%, measured by HPLC enantiomeric excess of 86.9%, Analytical conditions: column model Chiralcel OD-H, n has a volume ratio of the embankment and isopropanol 98: 2 analysis of wavelength 210 nm, the mobile phase flow rate of 1 mL / min, t! = 9.5 min, t 2 = 13.01 min, 86.9% ee.
IR (fi lm) 1760, 1731 cm- 1; ¾ NMR [400 MHz, CDC1 3] δ 7.46-7.44 (m, 2H), 7.36-7.31 (m, 8H), 3.99 (m, 3H), 0.96 (t , J = 7.2Hz, 3H); 13 C NMR [100 MHz, CDC1 3] δ 166.99, 138.98, 135.62, 128.67, 128.53, 128.36, 128.13, 127.04, 66.57, 62.16, 61.43, 13.96.
(2) (2S) -2-phenyl-3,3-hydroxy-3-methoxy propionate
The step (1) 162.56 g obtained in unpurified (2S) – 3,3-diphenyl acrylate epoxy crude compound was dissolved in 100 mL of methanol, 1 mL of boron trifluoride etherate ((2S ) – mole fraction of ethylene-3,3-diphenyl acrylate and boron trifluoride diethyl ether ratio of 1: 0.013) for the epoxy ring opening reaction; after controlling the reaction temperature is 20 ° C, reacted for 8 hours , the reaction solution was concentrated, ethyl acetate and aqueous extraction of the reaction solution after the ethyl acetate was concentrated to give 166.0 g of intermediate (2S) -2- hydroxy-3-methoxy-3,3-diphenyl acetic acid ester, crude yield of 92%, measured by high performance liquid enantiomeric excess of 86.9%, Analytical conditions: column model Chiralcel OD-H, n-and isopropyl alcohol embankment has a volume ratio of 98: 2, the wavelength analysis 210 nm, mobile phase flow rate of 1 mL / min,
min, t 2 = 14.51 min, 85.8% ee .;
IR (film) 1769, 1758 cm “1; 1H NMR [400 MHz, CDC1 3] δ 7.50-7.28 (m, 10H), 5.18 (s, 1H), 4.10 (t, 2H), 3.20 (s, J = 7.2Hz, 3H), 3.03 (s , 1H), 1.17 (t, J = 7.2Hz, 3H); 13 C NMR [100 MHz, CDC1 3] δ 172.48, 141.13, 140.32, 128.97, 128.73, 128.99, 127.81, 127.76, 127.62, 85.01, 77.42, 61.76, 52.62, 14.07.
-3-methoxy-3,3-diphenyl propionate (3) (2S) -2- [- oxo – dimethyl-pyrimidin-2-yl)]
Step (2) obtained in 166.0 g of intermediate (2S) -2- hydroxy-3-methoxy-3,3-diphenyl-propionate were added N, N- dimethylformamide 750 mL , potassium carbonate 45.54 g, was added 4,6-dimethyl after stirring for about half an hour 2-methanesulfonyl-pyrimidin nucleophilic substitution reaction at 80 ° C in an oil bath, the system, (2S) -2- hydroxy -3-methoxy-3,3-diphenyl-ethyl, 4,6-dimethyl-2 molar fraction ratio methylsulfonylpyrimidine and potassium carbonate is 1: 1.2: 0.6; nuclear magnetic after complete consumption of starting material was monitored after about 3 hours, water was added and the reaction solution was extracted with ethyl acetate, the ethyl acetate layer was concentrated to give 237.70 g of intermediate (2S) -2 – [(4,6- dimethyl-pyrimidin-2-yl ) – oxy] -3-methoxy-3,3-diphenyl propionate, crude yield greater than 99%, measured by HPLC enantiomeric excess of 85.9%, Analytical conditions: column Chiralcel OD model volume -H, isopropanol and n has embankment ratio of 98: 2, analysis wavelength was 210 nm, the mobile phase flow rate of 1 mL / min, t ^ lO.15 min, t 2 = 11.87 min, 85.9% ee .
IR (film) 1750cm “VH NMR [400 MHz, CDC1 3] δ 7.45 (d, J = 7.2 Hz, 2H), 7.39 (d, J = 7.2 Hz, 2H), 7.33-7.19 (m, 7H), 6.70 (s, 1H), 6.12 ( s, 1H), 4.01-3.85 (m, 2H), 3.50 (s, 3H) 2.38 (s, 6H), 0.93 (t, J = 6.8 Hz, 3H); 13 C NMR [100 MHz, CDC1 3] δ 169.51, 168.70, 163.86, 142.50, 141.29, 128.54, 128.03, 127.97, 127.94, 127.47, 127.40, 115.03, 83.76, 79.23, 77.43, 60.66, 53.92, 23.99, 13.93;. Anal Calcd For C 24 H 26 N 2 O 4 : C, 70.92; H, 6.45; N, 6.89 Found:. C, 70.72; H, 6.47; N, 6.83.
(4) (28) -2 – [(4,6-dimethyl-2-yl) – oxy] -3-methoxy-3,3-diphenyl-propionic acid ((+) – Abe Students Tanzania) preparation
To step (3) 237.7 g of the intermediate obtained (2S) -2 – [(4,6- dimethyl-pyrimidin-2-yl) – oxy] -3-methoxy-3,3-diphenyl propionate was dissolved in 1.2 L of organic solvent is 1,4-dioxane was added 600 mL of an aqueous solution containing 92.3 g of sodium hydroxide (wherein, (2S) -2 – [(4,6- dimethyl pyrimidin-2-yl) – oxy] -3-methoxy-3,3-diphenyl propionate and sodium hydroxide molar fraction ratio of 1: 4), the reaction temperature was 80 ° C, the reaction after 8 hours, the reaction solution was concentrated, using (1 L, 0.5 L, 0.5 L) and extracted with ether to remove organic impurities, the aqueous phase was extracted after addition of hydrochloric acid to adjust pH 3, large amount of solid appears; then the aqueous phase was added 1.0 L ethyl acetate, filtered to remove insolubles (insolubles which was found after analysis racemic ambrisentan, 23.37 g), the organic layer was concentrated, i.e., optically pure can be obtained 103.9 g (+) – ambrisentan, from 3 , 3-diphenyl acrylate departure, the optically pure (+) – ambrisentan, a yield of 52.3%. A small amount of the obtained reaction with ambrisentan diazo embankment derived (2S) -2 – [(4,6- dimethyl-pyrimidin-2-yl) – oxy] -3-methoxy-3,3 methyl diphenyl measured enantiomeric excess ambrisentan. (2S) -2 – [(4,6- dimethyl-pyrimidin-2-yl) – oxy] -3-methoxy-3,3-diphenyl-propionic acid methyl ester: HPLC measured enantiomer excess of 99.1%, Analytical conditions: column model Chiralcel OD-H, n has a volume ratio of isopropanol embankment 98:! 2, analysis wavelength was 210 nm, the mobile phase flow rate of 1 mL / min, t = 11.61 min, t 2 = 14.05 min , 99.1% ee.
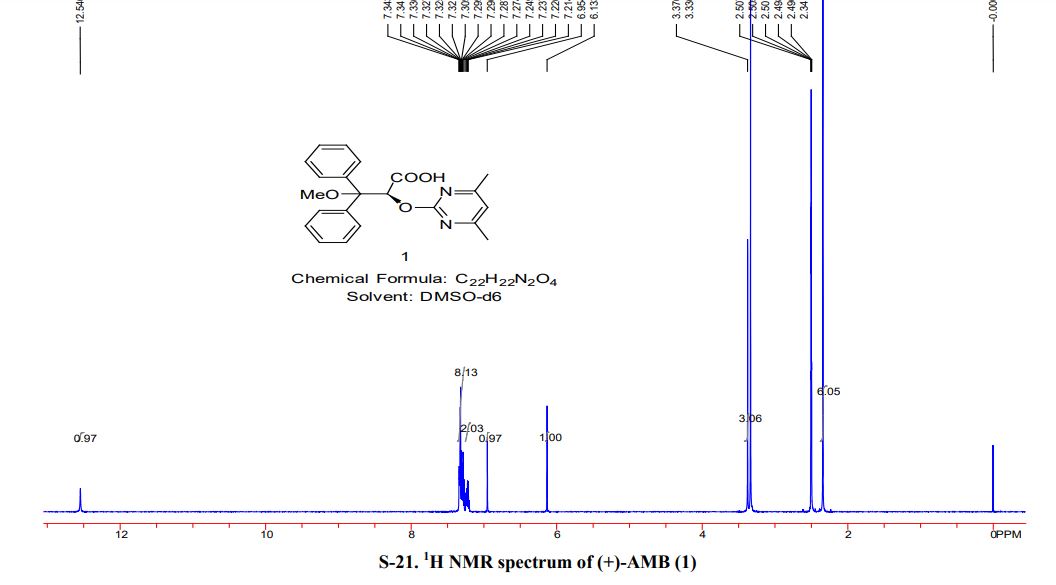
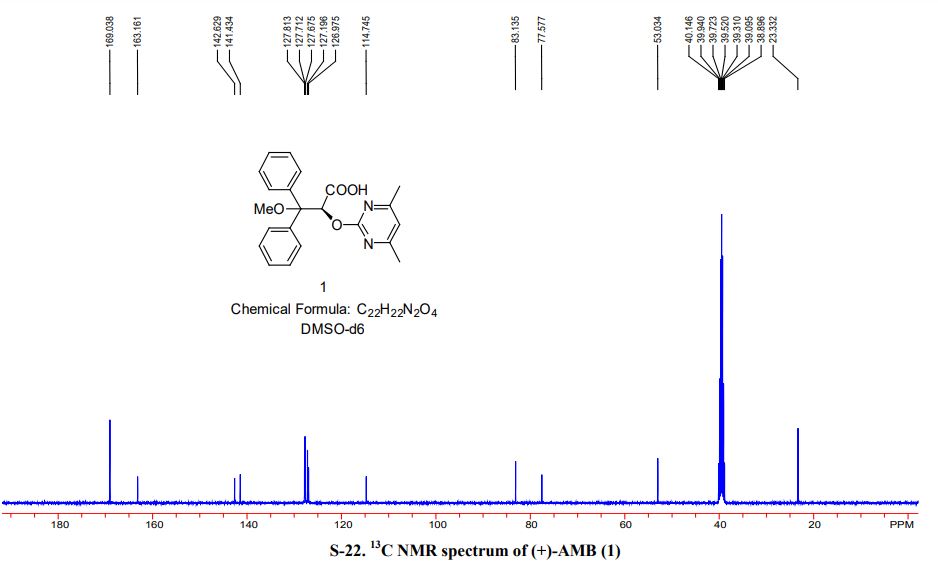
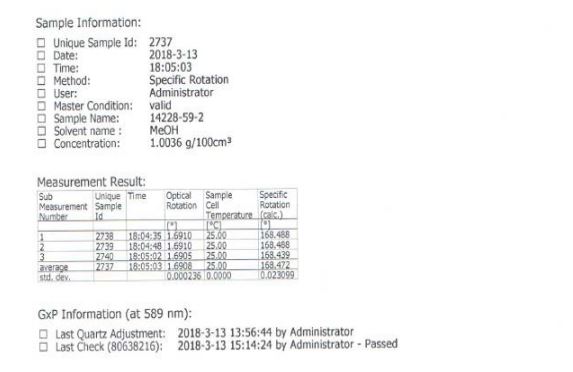
[a] D 25 = + 174.2 (c = 0.5, MeOH); mp> 150 ° C turns yellow,> 180 ° C into a black, 182 ° C melt; 1H NMR [400 MHz, CDC1 3] δ 7.43 ( d, J = Hz, 2H) , 7.29-7.19 (m, 8H), 6.63 (s, 1H), 6.30 (s, 1H), 3.26 (s, 3H) 2.31 (s, 6H); 13 C NMR [100 MHz, CDC1 3] δ 178.98,170.54, 169.70, 163.48, 139.91, 138.91, 128.77, 128.67, 128.22, 128.08, 115.34, 84.67, 77.55, 53.49, 23.93; 1H NMR [400 MHz, DMSO] δ 12.53 (s, 1H ), 7.34-7.20 (m, 10H) , 6.95 (s, 1H), 6.14 (s, 1H), 3.37 (s, 3H) 2.34 (s, 6H); 13 C NMR [100 MHz, DMSO] δ 169.01, 163.14, 142.59, 141.41, 127.80, 127.68, 127.64, 127.19, 126.95, 114.72, 83.12, 77.55, 52.99, 23.30.
CLIP
SEE https://www.pharmacodia.com/yaodu/html/v1/chemicals/a01610228fe998f515a72dd730294d87.html
CLIP
http://www.orgsyn.org/demo.aspx?prep=v89p0350#ref68
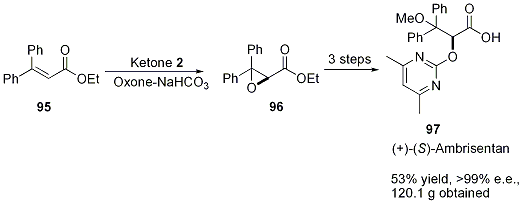
Shi and coworkers recently obtained 120 g of virtually enantiopure (+)-ambrisentan (97) without the need for column chromatography (Scheme 23).68 (+)-Ambrisentan, an endothelin-1 receptor antagonist, is currently used to treat hypertension. Ketone 2-catalyzed epoxidation afforded 96 in 90% conversion and 85% ee. Compound 97 was further enriched via precipitation and filtration of the racemate.
- Peng, X.; Li, P.; Shi, Y. J. Org. Chem. 2012, 77, 701-703.
Clip
https://pubs.acs.org/doi/10.1021/acs.oprd.8b00184
Process Research for (+)-Ambrisentan, an Endothelin-A Receptor Antagonist

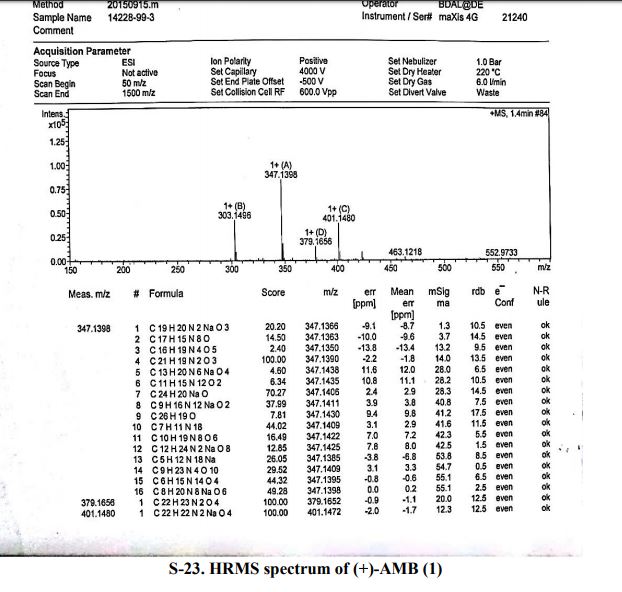
An efficient and robust synthetic route to (+)-ambrisentan ((+)-AMB) was designed by recycling the unwanted isomer from the resolution mother liquors. The racemization of AMB in the absence of either acid or base in the given solvents was reported. The recovery process was developed to produce racemates with purities over 99.5%. The mechanism of the formation of the process-related impurities of (+)-AMB is also discussed in detail. (+)-AMB was obtained in 47% overall yield with >99.5% purity and 99.8% e.e. by chiral resolution with only one recycling of the mother liquors on a 100-g scale without column purification.
https://pubs.acs.org/doi/suppl/10.1021/acs.oprd.8b00184/suppl_file/op8b00184_si_001.pdf
PaPER
https://pdfs.semanticscholar.org/3801/d5a98a526a4386c431e25d3ac99a328bfae2.pdf
CHEMICAL ENGINEERING TRANSACTIONS VOL. 46, 2015 A publication of The Italian Association of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Peiyu Ren, Yancang Li, Huiping Song Copyright © 2015, AIDIC Servizi S.r.l., ISBN 978-88-95608-37-2; ISSN 2283-9216
Improved Synthesis Process of Ambrisentan and Darusentan Jian Lia , Lei Tian*b, c a School of Environmental Science, Nanjing Xiaozhuang University, 3601 Hongjing Road, Nanjing, Jiangsu, 211171, China b School of Petroluem Engineer, Yangtze University, Wuhan, Hubei, 430100, P. R. China c Key Laboratory of Exploration Technologies for Oil and Gas Resources (Yangtze University), Ministry of Education tianlei4665@163.com
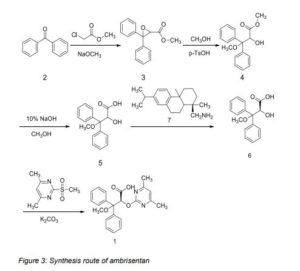
2-hydroxy-3-phenoxy-3, 3-diphenylpropinate (5) was prepared from benzophenone via Darzens, methanolysis and hydrolysis reaction. The compound (5) was salified with (S)-dehydroabietylamine (7) and diasterotropic resolution was carried out to provide the key intermediate (S)-2-hydroxy-3-methoxy-3, 3-diphenylpropionic acid (6). Compound (6) was condensed with 2-methylsulfonyl-4, 6-dimethylpyrimidine and 2-methoxysulfonyl4, 6-dimethylpyrimidine to afford ambrisentan (1) and darusentan (7), respectively. Two products were with excellent charity and chemical purity. The total yield of the synthesis was 30.1% and 29.6%, respectively.

Synthesis of methyl 3, 3-diphenyloxirane-2-carboxylate (3) To a solution of sodium methanolate (4.3 g, 79.6 mmol) in dry THF (25 mL) was added the solution of benzophenone (7.2 g, 39.5 mmol) and methyl chloroacetate (6.6 g, 60.8 mmol) in dry THF (15 mL) and stirred at -10 °C for 2 h. The mixture was quenched with water (50 mL). The solution was extracted with diethyl ether (80 mL×3). The organic phases were combined and washed with saturated NaCl. The solution was dried over Na2SO4, filtered, and evaporated under reduced pressure to afford a light yellow oil. The residue (3) can apply in next step without further purification (8.24 g, 82.1%). 1H-NMR (CDCl3): δ 3.52 (s, 3H), 3.99 (s, 1H), 7.32-7.45 (m, 10H).
Synthesis of 2-hydroxy-3-methoxy-3, 3-diphenylpropanoic acid (5)
To a solution of compound (3) (8.2 g, 31.6 mmol) in methanol (40 mL) was added p-toluene sulfonic acid (0.5 g) and stirred at for 0.5 h to afford the solution containing compound (4). Aqueous solution of NaOH (10% wt.) (60 mL) was added to the solution of compound (4) and the mixture was stirred at refluxed for 1h (ester disappeared by TLC). The solution was evaporated in order to remove a lot of methanol. The residue was acidified to pH 2 by conc. HCl. The solution was stirred for overnight and white solid stayed at the aqueous layer. The precipitate was filtered and deeply dried under vacuum to afford (5). (7.34 g, 85.3%). 1H-NMR (CDCl3): δ 3.22 (s, 3H), 5.14 (br, 1H), 5.20 (d, 1H), 7.18-7.37 (m, 10H), 12.30 (1H, br). Synthesis of (S)-2-hydroxy-3-methoxy-3, 3-diphenylpropanoate (6) The solution of compound (5) (14 g, 51.4 mmol) in methyltertiarybutylether (140 mL) was stirred and refluxed for 0.5 h. Dehydroabietylamine (7) (14.7 g, 51.4 mmol) in methyltertiarybutylether (50 mL) was added dropwise in 10 min. After addition, the reaction mixture was stirred for 1 h under reflux temperature. The reaction mixture was cooled to 0 °C and continued to stir for 2 h. The solid ((R, S)-diastereoisomers) was precipitated from the solution, filtered, washed with acetonitrile. The filtrate was diluted with water (100 mL) and acidified to pH 2 by conc. HCl. The aqueous solution was extracted with methylteriarybutylether (50 mL×4). The organic phases were combined and washed with water (80 mL). The organic phase was separated, dried over anhydrous Na2SO4 and evaporated under reduced pressure to afford white residue. The residue was recystallized from toluene to afford (6) as a white solid. (5.53 g, 39.5%). 1H-NMR (CDCl3): δ 3.22 (s, 3H), 5.14 (br, 1H), 5.20 (d, 1H), 7.18-7.37 (m, 10H), 12.30 (1H, br). [α] 20 D =12.3°(c=1.8% in ethanol).
Synthesis of (+)-ambrisentan (1) To a solution of compound (6) (3.6 g, 13.1 mmol) and NaNH2 (1.0 g, 25.6 mmol) in DMF (20 mL) was added 4, 6-dimethyl-2-(methylsulfonyl) pyrimidine (3.63 g, 19.6 mmol) in DMF (10 mL) slowly. After addition, the reaction was stirred for 5 h at room temperature. The solution was quenched with water (20 mL) and acidified to pH 2 by 10% H2SO4 aqueous solution. The mixture was extracted with ethyl acetate (50 mL ×4). The combined organic layers were washed with water (30 mL) and saturated NaCl solution (30 mL). The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure. The resulting residue was recrystallized from iso-propyl alcohol (30 mL) and water (40 mL), and precipitate formed was filtered off. The cake was deeply dried under vacuum to afford (1) as a white solid. (4.27 g, 86.1%). 1H-NMR (CDCl3): δ 2.39 (s, 6H), 3.32 (s, 3H), 6.43 (s, 1H), 6.70 (s, 1H), 7.28-7.40 (m, 8H), 7.53-7.56 (d, 2H). MS-EI (m/z): 377(M-H). HPLC (XDB-C18, CH3OH/10mmol/L NaH2PO4 + 0.1% H3PO4 = 70/30, 1.0 mL/min): tR 5.2 min (>99.0%); ee= 99.0%.
Patent EP2547663A1



References
- Jump up^ Vatter H, Seifert V (2006). “Ambrisentan, a non-peptide endothelin receptor antagonist”. Cardiovasc Drug Rev. 24 (1): 63–76. doi:10.1111/j.1527-3466.2006.00063.x. PMID 16939634.
- Jump up^ Frampton JE (2011). “Ambrisentan”. American Journal of Cardiovascular Drugs. 11 (4): 215–26. doi:10.2165/11207340-000000000-00000. PMID 21623643.
- Jump up^ Pollack, Andrew (2007-06-16). “Gilead’s Drug Is Approved to Treat a Rare Disease”. The New York Times. Archived from the original on May 24, 2013. Retrieved 2007-05-25.
- Jump up^ “U.S. Food and Drug Administration Approves Gilead’s Letairis Treatment of Pulmonary Arterial Hypertension” (Press release). Gilead Sciences. 2007-06-15. Archived from the original on 2007-09-27. Retrieved 2007-06-16.
- Jump up^ “FDA Approves New Orphan Drug for Treatment of Pulmonary Arterial Hypertension” (Press release). Food and Drug Administration. 2007-06-15. Archived from the original on 23 June 2007. Retrieved 2007-06-22.
- Jump up^ “GlaxoSmithKline’s Volibris (ambrisentan) receives authorisation from the European Commission for the treatment of Functional Class II and III Pulmonary Arterial Hypertension” (Press release). GlaxoSmithKline. 2008-04-25. Archived from the original on 30 April 2008. Retrieved 2008-04-29.
- Jump up^ Waknine, Yael (2005-05-09). “International Approvals: Ambrisentan, Oral-lyn, Risperdal”. Medscape. Retrieved 2007-06-16.
- Jump up^ de Raaf MA, Beekhuijzen M, Guignabert C, Vonk Noordegraaf A, Bogaard HJ (2015). “Endothelin-1 receptor antagonists in fetal development and pulmonary arterial hypertension”. Reproductive Toxicology. 56: 45–51. doi:10.1016/j.reprotox.2015.06.048. PMID 26111581.
- Jump up^ Galiè, Nazzareno; Barberà, Joan A.; Frost, Adaani E.; Ghofrani, Hossein-Ardeschir; Hoeper, Marius M.; McLaughlin, Vallerie V.; Peacock, Andrew J.; Simonneau, Gérald; Vachiery, Jean-Luc; Grünig, Ekkehard; Oudiz, Ronald J.; Vonk-Noordegraaf, Anton; White, R. James; Blair, Christiana; Gillies, Hunter; Miller, Karen L.; Harris, Julia H.N.; Langley, Jonathan; Rubin, Lewis J. (2015). “Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension”. New England Journal of Medicine. 373 (9): 834–44. doi:10.1056/NEJMoa1413687.
- US5703017
- CA2201785
- US5840722
- US7601730
- US8377933
- US7109205
- USRE42462
- US8349843
- US9474752
- US9549926
Patent
Non-Patent Citation
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Undetermined |
| Protein binding | 99% |
| Elimination half-life | 15 hours (terminal) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.184.855 |
| Chemical and physical data | |
| Formula | C22H22N2O4 |
| Molar mass | 378.421 g/mol |
| 3D model (JSmol) | |
/////////////Ambrisentan, أمبريسنتان , 安立生坦 , BSF-208075, LU-208075, アンブリセンタン
CC1=CC(=NC(=N1)OC(C(=O)O)C(C2=CC=CC=C2)(C3=CC=CC=C3)OC)C