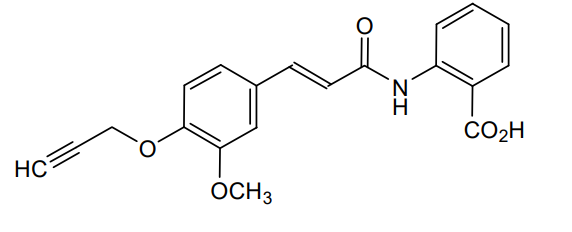
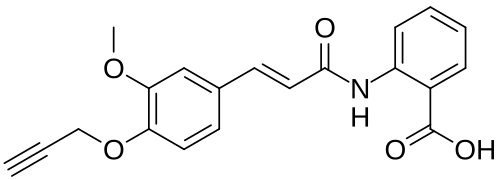
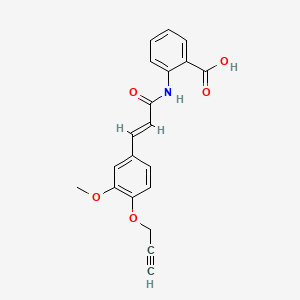
Asengeprast
CAS 1001288-58-9
FT011, FT 011, orphan drug status, systemic sclerosis, SHP-627, SHP 627,
Fast Track
2-[[(E)-3-(3-methoxy-4-prop-2-ynoxyphenyl)prop-2-enoyl]amino]benzoic acid
2-[(2E)-3-{3-methoxy-4-[(prop-2-yn-1-yl)oxy]phenyl}prop-2-enamido]benzoic acid G protein-coupled receptor 68 (GPR68) antagonist,
anti-inflammatory
MF C20H17NO5 MW 351.4 g/mol. C6V7ZU2NPR
Asengeprast (development code FT011) is an experimental scleroderma drug candidate.[1] It is a small molecule inhibitor of the G-protein coupled receptor GPR68 with antifibrotic activity.[2] It is being developed by Certa Therapeutics.
The European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) has granted orphan drug status to FT011, for systemic sclerosis (SSc).[3]
Asengeprast has been reported to attenuate fibrosis and chronic heart failure in experimental diabetic cardiomyopathy.[4] Asengeprast can also inhibit kidney fibrosis and prevent kidney failure.[5] It was developed by structure-activity optimization of the antifibrotic activity of cinnamoyl anthranilates, by assessment of their ability to prevent TGF-beta-stimulated production of collagen.[6]
Effects of FT011 in Systemic Sclerosis, CTID: NCT04647890
Phase: Phase 2, Status: Completed, Date: 2023-12-20
SYN
Evaluation and optimization of antifibrotic activity of cinnamoyl anthranilates
Publication Name: Bioorganic & Medicinal Chemistry Letters
Publication Date: 2009-12-15
PMID: 19879136
DOI: 10.1016/j.bmcl.2009.09.120
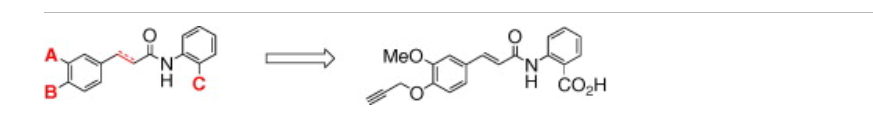
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018144620&_cid=P21-MFTHV7-45829-1
PAT
Publication Number: WO-2008003141-A1
Priority Date: 2006-07-05
- Tranilast analogues (substituted cinnamoyl anthranilate compounds) for treatment of conditions associated with firbrosisPublication Number: NZ-574028-APriority Date: 2006-07-05
- Therapeutic CompoundsPublication Number: US-2010130497-A1Priority Date: 2006-07-05
- Therapeutic compoundsPublication Number: US-2014357628-A1Priority Date: 2006-07-05
- Therapeutic compoundsPublication Number: US-8765812-B2Priority Date: 2006-07-05Grant Date: 2014-07-01
- Therapeutic compoundsPublication Number: US-9561201-B2Priority Date: 2006-07-05Grant Date: 2017-02-07



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- “Asengeprast Ligand page”. IUPHAR/BPS Guide to PHARMACOLOGY.
- “Certa Therapeutics website”.
- Inácio P (23 July 2024). “Certa’s FT011 granted orphan drug status in Europe for SSc”. Scleroderma News.
- Zhang Y, Edgley AJ, Cox AJ, Powell AK, Wang B, Kompa AR, et al. (May 2012). “FT011, a new anti-fibrotic drug, attenuates fibrosis and chronic heart failure in experimental diabetic cardiomyopathy”. European Journal of Heart Failure. 14 (5): 549–562. doi:10.1093/eurjhf/hfs011. PMID 22417655.
- Gilbert RE, Zhang Y, Williams SJ, Zammit SC, Stapleton DI, Cox AJ, et al. (2012). “A purpose-synthesised anti-fibrotic agent attenuates experimental kidney diseases in the rat”. PLOS ONE. 7 (10): e47160. Bibcode:2012PLoSO…747160G. doi:10.1371/journal.pone.0047160. PMC 3468513. PMID 23071743.
- Zammit SC, Cox AJ, Gow RM, Zhang Y, Gilbert RE, Krum H, et al. (December 2009). “Evaluation and optimization of antifibrotic activity of cinnamoyl anthranilates”. Bioorganic & Medicinal Chemistry Letters. 19 (24): 7003–7006. doi:10.1016/j.bmcl.2009.09.120. PMID 19879136.
| Chemical structure of asengeprast (FT011) | |
| Clinical data | |
|---|---|
| Other names | FT011 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1001288-58-9 |
| PubChem CID | 23648966 |
| ChemSpider | 24664633 |
| UNII | C6V7ZU2NPR |
| ChEMBL | ChEMBL1075834 |
| Chemical and physical data | |
| Formula | C20H17NO5 |
| Molar mass | 351.358 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
- FT011, a Novel Cardiorenal Protective Drug, Reduces Inflammation, Gliosis and Vascular Injury in Rats with Diabetic RetinopathyPublication Name: PLOS ONEPublication Date: 2015-07-29PMCID: PMC4519240PMID: 26222724DOI: 10.1371/journal.pone.0134392
- A new anti-fibrotic drug attenuates cardiac remodeling and systolic dysfunction following experimental myocardial infarctionPublication Name: International Journal of CardiologyPublication Date: 2013-09-30PMID: 23219315DOI: 10.1016/j.ijcard.2012.11.067
- Attenuation of Armanni–Ebstein lesions in a rat model of diabetes by a new anti-fibrotic, anti-inflammatory agent, FT011Publication Name: DiabetologiaPublication Date: 2012-12-16PMID: 23242170DOI: 10.1007/s00125-012-2805-9
- A Purpose-Synthesised Anti-Fibrotic Agent Attenuates Experimental Kidney Diseases in the RatPublication Name: PLoS ONEPublication Date: 2012-10-10PMCID: PMC3468513PMID: 23071743DOI: 10.1371/journal.pone.0047160
- FT011, a new anti‐fibrotic drug, attenuates fibrosis and chronic heart failure in experimental diabetic cardiomyopathyPublication Name: European Journal of Heart FailurePublication Date: 2012-05PMID: 22417655DOI: 10.1093/eurjhf/hfs011
///////////Asengeprast, FT011, FT 011, orphan drug status, systemic sclerosis, SHP-627, SHP 627, C6V7ZU2NPR, Fast Track














