
Carglumic acid
N-Carbamyl-L-glutamate;
- Molecular FormulaC6H10N2O5
- Average mass190.154 Da
Carglumic acid is a Carbamoyl Phosphate Synthetase 1 Activator. The mechanism of action of carglumic acid is as a Carbamoyl Phosphate Synthetase 1 Activator.
For the treatment of acute and chronic hyperammonaemia in patients with N-acetylglutamate synthase (NAGS) deficiency. This enzyme is an important component of the urea cycle to prevent build up of neurotoxic ammonium in the blood.
EMA
Carglumic acid exists as a white powder or colourless crystals. It is soluble in boiling water, slightly soluble in cold water and practically insoluble in organic solvents (cyclohexane, dichloromethane, ether). The water solubility of carglumic acid at pH 2.0 is 21.0 g/L. It increases rapidly between the pH 3.0 (28.2 g/L) and the pH 5.0 (440.9 g/L). The solubility of carglumic acid in water is stable between pH 6.0 (555.5 g/L) and pH 8.0 (553.9 g/L). Carglumic acid is prepared from L-glutamic acid. It exhibits stereoisomerism due to the presence of one chiral centre and has one optical isomer; N-carbamoyl-D-glutamic acid.
ORIGINATOR ORPHAN EUROPE
POLA CHEMICAL
ORPHAN DRUG
EU APPROVED 2003 ORPHAN EUROPE
FDA 2010 ORPHAN EUROPE
JAPAN 2016 POLA CHEM
CARBAGLU®
(carglumic acid) Tablet for Oral Suspension
DESCRIPTION
CARBAGLU tablets for oral suspension, contain 200 mg of carglumic acid. Carglumic acid, the active substance, is a Carbamoyl Phosphate Synthetase 1 (CPS 1) activator and is soluble in boiling water, slightly soluble in cold water, and practically insoluble in organic solvents.
Chemically carglumic acid is N-carbamoyl-L-glutamic acid or (2S)-2-(carbamoylamino) pentanedioic acid, with a molecular weight of 190.16.
The structural formula is:
 |
Molecular Formula: C6H10N2O5
The inactive ingredients of CARBAGLU are croscarmellose sodium, hypromellose, microcrystalline cellulose, silica colloidal anhydrous, sodium lauryl sulfate, sodium stearyl fumarate.
Carglumic Acid is an orally active, synthetic structural analogue of N-acetylglutamate (NAG) and carbamoyl phosphate synthetase 1 (CPS 1) activator, with ammonia lowering activity. NAG, which is formed by the hepatic enzyme N-acetylglutamate synthase (NAGS), is an essential allosteric activator of the enzyme carbamoyl phosphate synthetase 1 (CPS 1). CPS 1 plays an essential role in the urea cycle and converts ammonia into urea. Upon oral administration, carglumic acid can replace NAG in NAGS deficient patients and activates CPS 1, which prevents hyperammonaemia.
Carglumic acid is an orphan drug, marketed by Orphan Europe under the trade name Carbaglu. Carglumic acid is used for the treatment of hyperammonaemia in patients with N-acetylglutamate synthase deficiency.[1][2] The initial daily dose ranges from 100 to 250 mg/kg, adjusted thereafter to maintain normal plasma levels of ammonia.
The US FDA approved it for treatment of hyperammonaemia on March 18, 2010. Orphan Drug exclusivity expired on March 18, 2017.[3]
USFDA
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022562s000chemr.pdf
Carbaglu (carglumic acid) Tablets 200 mg, is a white elongated tablet with three score marks on both sides engraved C’s on one side. It is a dispersible tablet designed to be dispersed in of water and ingested or administered through a syringe via a nasogastric tube. It is indicated for treatment of acute hyperammonemia in patients with NAGS deficiency.
The drug substance, carglumic acid, is an allosteric activator of a critical urea cycle enzyme, carbamoyl phosphate synthetase (CPS). It is a close analog of the naturally occurring activator, N-acetyl glutamate (NAG). Carglumic acid is a urea-like derivative of the amino acid L-glutamate and contains one chiral center. The drug substance solid form is the neutral dicarboxylic acid and is a white crystalline powder. The water solubility of the drug substance depends on the . polymorphic solid form has been found.
The drug substance is manufactured by .
The facility was found to have acceptable cGMP status during an inspection by
the Agency in November 2009. The synthesis of carglumic acid consists of a
Regarding characterization, the drug substance structure was determined by
NMR, MS, IR and Regarding impurities, two potential
impurities are possible due to
hydantoin-5-proprionic acid (HPA) and diaza-1,3-dione-2,4-carboxy-7-
cycloheptane (Diaza). Only the has been detected at
batch release and it increases in amount during storage at elevated temperatures
but not at room temperature. This impurity also increases during drug product
storage at room temperature but not at refrigerated temperatures, see above
discussion. The starting materials, , were not
detected in several batches and therefore routine testing is not required.
Regarding drug substance specification, identity testing is by IR and HPLC.
Other tests include optical rotation, melting point, pH of 0.5% solution, loss on
drying, residue on ignition, heavy metals, assay and impurities by HPLC.
Regarding chiral purity, the observed specific optical rotation is small and
therefore not a very precise method for determination of chiral purity. Although
a chiral HPLC method was developed, since the r was not detected in
any samples (the limit of detection was 0.1%) during the development, originally
the sponsor did not propose to implement the test in the specification. However,
the Agency recommended that the chiral HPLC method be included in the
specification to assure chiral purity, and the sponsor agreed to do so with the limit
for the NMT
Batch release data were provided that justified the proposed acceptance limits. In
general, measured total impurities were low in the drug substance, about .
Appropriate in-house reference standards were established.
Stability results for 3 batches stored at 25°C/60%RH for 36 months remained
within the tight specification limits. A re-test period of for the drug
substance stored in its original packaging at room temperature is granted.
B. Description of How the Drug Product is Intended to be Used
The drug product tablets may be dispersed in a minimum amount of water
mL per tablet) and ingested immediately or administered through a syringe via a
nasogastric tube. The suspension has a slightly acidic taste.
NDA 022562
EUROPE
21 April 2017 EMA/CHMP/404487/2017 Committee for Medicinal Products for Human Use (CHMP) Assessment report Ucedane International non-proprietary name: carglumic acid Procedure No. EMEA/H/C/004019/0000
Carglumic acid (also called N-carbamyl-L-glutamate, or carbamylglutamate) is an orally active deacylaseresistant synthetic structural N-acetylglutamate (NAG) analogue. NAG, which is formed by the hepatic enzyme N-acetylglutamate synthase (NAGS), is an essential allosteric activator of the enzyme carbamoyl phosphate synthetase 1 (CPS-1). CPS-1 plays an essential role in the urea cycle and converts ammonia into urea which prevents hyperammonaemia. Despite a lower affinity of carbamoyl phosphate synthetase for carglumic acid than for N-acetylglutamate, carglumic acid has been shown in vivo to stimulate carbamoyl phosphate synthetase and to be much more effective than N-acetylglutamate in protecting against ammonia intoxication in rats.
Carglumic acid was first authorised in the EU as Carbaglu dispersible tablets in January 2003. At the time of approval Carbaglu was indicated for the treatment of hyperammonaemia associated with N-acetylglutamate synthase deficiency. Subsequently, the approved indications for Carbaglu have been extended and is now also authorised for the treatment of hyperammonaemia due to, isovaleric acidaemia, methymalonic acidaemia, or propionic acidaemia. Ucedane is indicated in treatment of hyperammonaemia due to N-acetylglutamate synthase primary deficiency. Proposed posology and method of administration for Ucedane
The chemical name of the active substance, carglumic acid, is N-Carbamyl-L-glutamic acid corresponding to the molecular formula C6H10N2O5. It has a relative molecular mass 190.16 g/mol and the following structure:
Carglumic acid exists as a white powder or colourless crystals. It is soluble in boiling water, slightly soluble in cold water and practically insoluble in organic solvents (cyclohexane, dichloromethane, ether). The water solubility of carglumic acid at pH 2.0 is 21.0 g/L. It increases rapidly between the pH 3.0 (28.2 g/L) and the pH 5.0 (440.9 g/L). The solubility of carglumic acid in water is stable between pH 6.0 (555.5 g/L) and pH 8.0 (553.9 g/L). Carglumic acid is prepared from L-glutamic acid. It exhibits stereoisomerism due to the presence of one chiral centre and has one optical isomer; N-carbamoyl-D-glutamic acid.
Adverse effects
The most common adverse effects include vomiting, abdominal pain, fever, and tonsillitis.[4]
SYNTHESIS PHARMACODIA
http://en.pharmacodia.com/web/drug/1_468.html

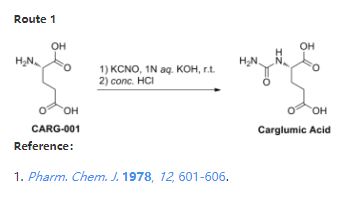
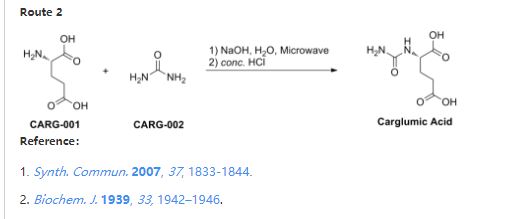
References
- Jump up^ Caldovic L, Morizono H, Daikhin Y, Nissim I, McCarter RJ, Yudkoff M, Tuchman M (2004). “Restoration of ureagenesis in N-acetylglutamate synthase deficiency by N-carbamylglutamate”. J Pediatr. 145 (4): 552–4. doi:10.1016/j.jpeds.2004.06.047. PMID 15480384.
- Jump up^ Elpeleg O, Shaag A, Ben-Shalom E, Schmid T, Bachmann C (2002). “N-acetylglutamate synthase deficiency and the treatment of hyperammonemic encephalopathy”. Ann Neurol. 52 (6): 845–9. doi:10.1002/ana.10406. PMID 12447942.
- Jump up^ “Patent and Exclusivity Search Results”.
- Jump up^ Drugs.com: Professional Drug Facts for Carglumic Acid.
 |
|
| Clinical data | |
|---|---|
| Synonyms | (S)-2-ureidopentanedioic acid |
| AHFS/Drugs.com | Consumer Drug Information |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Protein binding | Undetermined |
| Metabolism | Partial |
| Elimination half-life | 4.3 to 9.5 hours |
| Excretion | Fecal (60%) and renal (9%, unchanged) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.116.323 |
| Chemical and physical data | |
| Formula | C6H10N2O5 |
| Molar mass | 190.2 g/mol |
////////////////Carglumic acid, FDA 2010, карглумовая кислота , حمض كاروغلوميك , カルグルミ酸 , ORPHAN, ORPHAN EU, JAPAN 2016, EU 2003, POLA, ORPHAN, OE 312
C(CC(=O)O)C(C(=O)O)NC(=O)N















