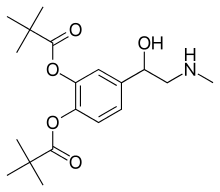
Dipivefrine
- Molecular FormulaC19H29NO5
- Average mass351.437 Da
Dipivefrine (INN) or dipivefrin (USAN), trade name Propine among others, is a prodrug of epinephrine, and is used to treat open-angle glaucoma.[1][2] It is available as a 0.1% ophthalmic solution. It is no longer available in the United States.[3]
Dipivefrin is a prodrug with little or no pharmacologically activity until it is hydrolyzed into epinephrine inside the human eye. The liberated epinephrine, an adrenergic agonist, appears to exert its action by stimulating α -and/or β2-adrenergic receptors, leading to a decrease in aqueous production and an enhancement of outflow facility. The dipivefrin prodrug delivery system is a more efficient way of delivering the therapeutic effects of epinephrine, with fewer side effects than are associated with conventional epinephrine therapy. Dipivefrin is used as initial therapy for the control of intraocular pressure in chronic open-angle glaucoma.

Contraindications
Use in narrow-angle glaucoma may be dangerous because it could make the eye susceptible to an attack of angle closure,[2] causing an increase in pressure and pain, and possibly loss of vision.
Side effects
The most common side effects of dipivefrine are burning, stinging and other irritations of the eye. Possible, but uncommon, side effects are those of epinephrine: tachycardia (fast heartbeat), hypertension (high blood pressure) and arrhythmias (irregular heartbeat).[2]
Pharmacology
Dipivefrine penetrates the cornea and is then hydrolysed to epinephrine by esterase enzymes. It increases outflow of the aqueous humour and also reduces its formation (mediated by its action on α1 and α2 receptors), thus reducing pressure inside the eye. It also increases the conductivity of trabecular filtering cells (a β2 receptor mediated action). It is preferred to epinephrine because it is longer acting, more consistent in its action and better tolerated.[1]
Patent
https://patents.google.com/patent/CN102153485A/en

Example 1 [0023] Embodiment
[0024] A 600g (3. 21mol) 4_ chloroacetyl catechol, the IOL 6L methylene chloride was added 4-neck flask, the system was cooled to 5 ° C, was added 666g (6. 58mol) of triethylamine, and then was added dropwise 784g (6. 5mol) pivaloyl chloride was added dropwise and stirring was continued after the pool. Filtered off with suction, the filtrate by rotary evaporation; to give 990g yellow-brown solid, 4- (2-chloroacetyl) -1,2-pivalate phenyl ester, the content of 96.2%. [0025] The 35mol) N- methyl amine section, 370g (3. 66mol) of triethylamine, 25g (0. 15mol) KI, 3L DMF was added 4-neck flask of the IOL. Cooled to 0 ° C, was added dropwise 990g (2. 8mol) 4- (2- chloroacetyl) -I, DMF solution tank 2-phenyl pivalate ester. At room temperature was stirred for 4h.
[0026] suction filtration, washed with water IOL filtrate was added 3 times, the organic phase was separated, the organic phase by rotary evaporation to give a yellow-brown oil; frozen stirring, the precipitated solid was suction filtered to give a solid 923. Og. I.e., 1- (3,4-pivaloyloxymethyl-phenyl) -2- (N- benzyl-methylamino) -1-one content of 96.5%.
[0027] Take 625g (1. 422mol) 1_ (3,4- two pivaloyloxymethyl phenyl) _2_ (N- benzyl-methylamino) ketone, 6L IOL of absolute ethanol was added 4-neck flask. Under cooling, was added 65g (1.71mol) of sodium borohydride. At room temperature was stirred for 4h. 500mL of water was slowly added to the system, then add ethyl acetate extract products. After solvent removal to give 552. 5g of solid particles, i.e. 1_ (3, 4-pivaloyloxymethyl-phenyl) -2- (N- benzyl-methylamino) ethanol, the content of 98.2%.
[0028] 1828 was added to the beaker (0.41211101) of 1- (3,4-pivaloyloxymethyl-phenyl) -2 – (^ -benzyl methylamino) ethanol, with ethanol and dissolved IL; to 2L autoclave was charged with 13g 5% palladium on carbon, infiltration system with IOOml ethanol, then added to the solution in a closed system. Through hydrogenation under hydrogen 2MPa pool.
[0029] suction filtered to remove palladium on carbon. The filtrate was twice filtered off with suction, the filtrate by rotary evaporation to give a yellow-brown oil; standing crystallization, the precipitated pale yellow solid was suction filtered to give a solid crude product.
[0030] After the solution was washed with methanol hydrochloride salt to give an off-white solid 119. 9g, dipivefrin i.e., the content of 98.9%.
[0031] m.p. 161 ~162 ° C;
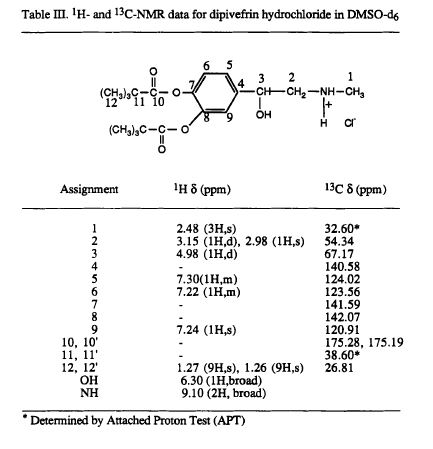
[0032] 1H NMR (CDCl3) δ: 1. 35 (s, 18Η), 2 68 (s, 3Η), 3 07-3 13 (m, 2Η), 5 36-5 39 (m….. , 1H),
[0033] 7. 06-7. 30 (m, 3H), 8. 61 (s, 1H), 9. 48 (s, 1H)
Dipivefrin prepared: Example 2 [0034] Embodiment
[0035] A 600g (3. 21mol) 4_ chloroacetyl catechol, the IOL 6L methylene chloride was added 4-neck flask, the system was cooled to 10 ° C, was added 666g (6. 58mol) of triethylamine, and then dropwise 78½ (6. 5mol) pivaloyl chloride was added dropwise and stirring was continued after the pool. Filtered off with suction, the filtrate by rotary evaporation; 978. 2g to give yellow-brown solid, 4- (2-chloroacetyl) -1,2-pivalate phenyl ester, the content of 96. 2% o
[0036] The 35mol) N- methyl amine section, 370g (3. 66mol) of triethylamine, 25g (0. 15mol) KI, 3L DMF was added 4-neck flask of the IOL. Cooled to O0C, dropwise 978. 2g (2. 77mol) 4- (2- chloroacetyl) of DMF solution tank Laid-1,2-phenyl valerate. At room temperature was stirred for 4h.
[0037] suction filtration, washed with water IOL filtrate was added 3 times, the organic phase was separated, the organic phase by rotary evaporation to give a yellow-brown oil; frozen stirring, the precipitated solid was suction filtered to give a solid 910. 2g. I.e., 1- (3,4-pivaloyloxymethyl-phenyl) -2- (N- benzyl-methylamino) -1-one content of 96.3%.
[0038] Take 625g (1. 422mol) 1_ (3,4- two pivaloyloxymethyl phenyl) _2_ (N- benzyl-methylamino) ketone, 6L IOL of absolute ethanol was added 4-neck flask. Under cooling, was added 97g (l. SOmol) potassium borohydride. Stirred cell at room temperature. 500mL of water was slowly added to the system, then add ethyl acetate extract products. After solvent removal to give 532. 7g of solid particles, i.e. 1_ (3, 4-pivaloyloxymethyl-phenyl) -2- (N- benzyl-methylamino) ethanol, the content of 98.0%.
[0039] 1828 was added to the beaker (0.41211101) of 1- (3,4-pivaloyloxymethyl-phenyl) -2 – (^ -benzyl methylamino) ethanol, with ethanol and dissolved IL; to 2L autoclave was charged with 15g 5% palladium on carbon, infiltration system with IOOml ethanol, then added to the solution in a closed system. Through hydrogenation under hydrogen 2MPa pool.
[0040] suction filtered to remove palladium on carbon. The filtrate was twice filtered off with suction, the filtrate by rotary evaporation to give a yellow-brown oil; standing crystallization, the precipitated pale yellow solid was suction filtered to give a solid crude product.
[0041] After the solution was washed with methanol hydrochloride salt to give an off-white solid was 112. 8g, i.e., dipivefrin, content 98.6%.
3 [0042] Example 2: Preparation of dipivefrin
[0043] A 600g (3. 21mol) 4_ chloroacetyl catechol, the IOL 6L methylene chloride was added 4-neck flask, the system was cooled to 5 ° C, was added 897g (6. 5mol) of potassium carbonate, and then drops was added 784g (6. 5mol) pivaloyl chloride addition was completed stirring was continued Syndrome. Filtered off with suction, the filtrate by rotary evaporation; to give 900g yellow-brown solid, 4- (2-chloroacetyl) -1,2-pivalate phenyl ester, the content of 95.6%.
[0044] A 526g (4. 35mol) N_ methylbenzylamine, 414g (3. Omol) of potassium carbonate, 25g (0. 15mol) KI, 3L DMF force Λ IOL of four port flask. Cooled to O0C, was added dropwise 900g (2. 55mol) 4- (2- chloroacetyl) of DMF solution of 1,2-Shan Laid phenyl valerate. It was stirred at room temperature Mi.
[0045] The suction filtration, washed with water IOL filtrate was added 3 times, the organic phase was separated, the organic phase by rotary evaporation to give a yellow-brown oil; frozen stirring, the precipitated solid was suction filtered to give a solid 820g. I.e., 1- (3,4-pivaloyloxymethyl-phenyl) -2- (N- benzyl-methylamino) -1-one content of 95.6%.
[0046] Take 625g (1. 42mol) 1_ (3,4- two pivaloyloxymethyl phenyl) _2_ (N- benzyl-methylamino) ketone, 6L IOL of absolute ethanol was added 4-neck flask. Under cooling, was added 65g (1.71mol) of sodium borohydride. Stirred cell at room temperature. 500mL of water was slowly added to the system, then add ethyl acetate extract products. After solvent removal to give 512. 5g of solid particles, i.e. 1_ (3, 4-pivaloyloxymethyl-phenyl) -2- (N- benzyl-methylamino) ethanol, the content of 98.0%.
[0047] 1828 was added to the beaker (0.41211101) of 1- (3,4-pivaloyloxymethyl-phenyl) -2 – (^ -benzyl methylamino) ethanol, with ethanol and dissolved IL; to 2L autoclave was charged with 16g 5% palladium on carbon, infiltration system with IOOml ethanol, then added to the solution in a closed system. Through hydrogenation under hydrogen 2MPa pool.
[0048] suction filtered to remove palladium on carbon. The filtrate was twice filtered off with suction, the filtrate by rotary evaporation to give a yellow-brown oil; standing crystallization, the precipitated pale yellow solid was suction filtered to give a solid crude product.
[0049] After the solution was washed with methanol hydrochloride salt to give an off-white solid was 109. 8g, i.e., dipivefrin, content 98.5%.
SYN
SYN
2-chloro-3′,4′-dihydroxyacetophenone, 99-40-1
3′,4′-dihydroxy-2-methylaminoacetophenone, 99-45-6
2,2-dimethylpropanoic acid 4-[(methylamino)acetyl]-1,2-phenylene ester, 52245-00-8
Pivaloyl chloride, 3282-30-2
Trimethylacetyl chloride, 3282-30-2
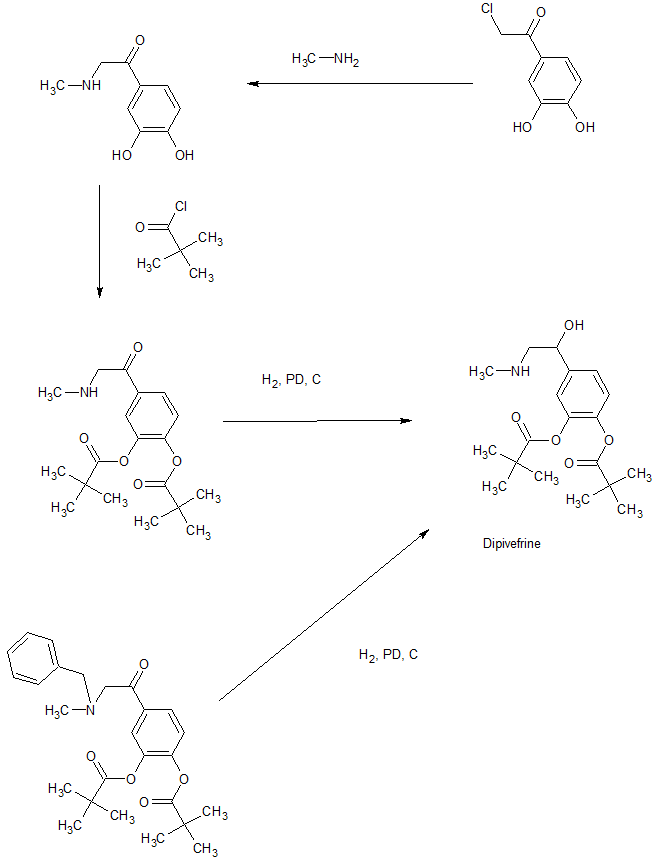
1-(3,4-dipivaloyloxyphenyl)-2-(benzylmethylamino)ethan-1-one, 42146-03-2
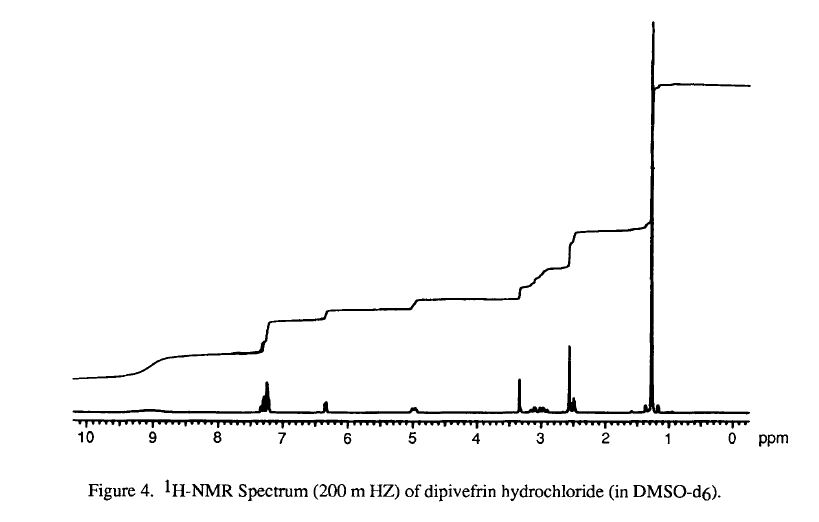
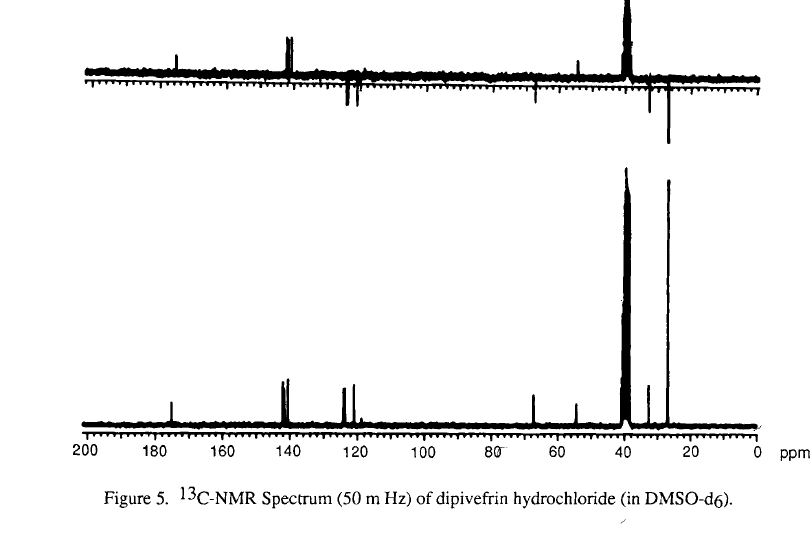
SPECTROSCOPY
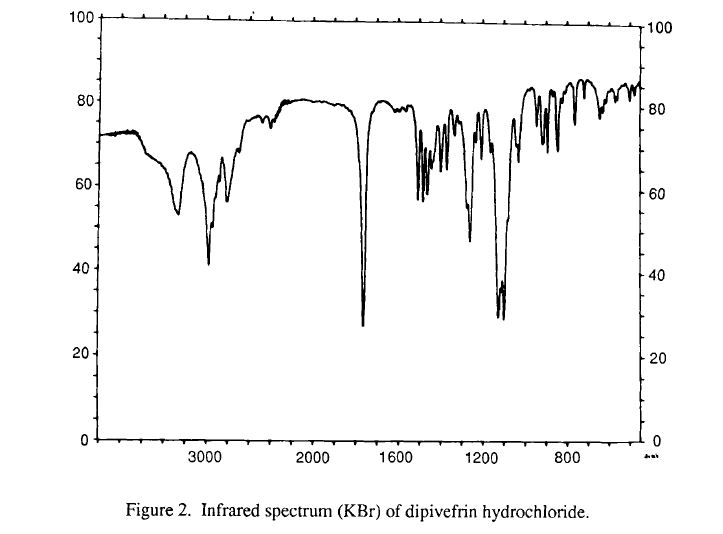
infrared spectral assignments for dipiveh hydrochloride
Wavelength (cm-1) Assignment
3255,2804,2475, 2397 RflHz+-NH stretch
2974-2875 sp3 C-H stretch
1273, 1258-1163 C-0-C stretch
3600-3400 0-H stretch
phenyl ester C=O stretch 1761
aromatic C-C stretch 1614, 1595, 1562, 1504
sp3 C-H bending and scissoring 1481, 1461, 1441, 1397
tert-butyl C-H bending1368, 1332
secondary alcohol C-0 stretch 1 124- 1028
out-of-plane bending for 1,substituted benzene ring 3,4 891,842
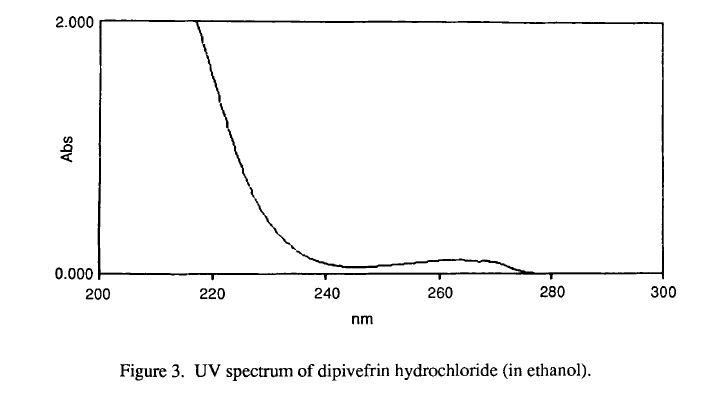
Ultraviolet absorption of dipivefrin hydrochloride
E (176, 1 cm)
Solvent 210 nm 264 Nn 270 nm
Acetonitrile 267.3 14.8 13.4
Ethanol 246.8 14.5 13.1
pH 3 Buffer 266.7 12.4 10.4
pH 7 Buffer 257.6 10.8 8.9
Water 278.0 18.0 16.2

References
- ^ Jump up to:a b KD Tripari. Essentials of Medical Pharmacology (5 ed.). Jaypee Brothers Medical Publishers(P) Ltd. p. 88. ISBN 81-8061-187-6.
- ^ Jump up to:a b c Dipivefrin FDA Professional Drug Information.
- ^ Zhang L, Weizer JS, Musch DC (2017). “Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty”. Cochrane Database Syst Rev. 2: CD010746. doi:10.1002/14651858.CD010746.pub2. PMC 5477062. PMID 28231380.
-
- Hussain, A.; Truelove, J.E.: J. Pharm. Sci. (JPMSAE) 65, 1510 (1976).
- US 3 839 584.
- a DOS 2 343 657 (Interx Res. Corp.; appl. 30.8.1973; USA-prior. 31.8.1972).
- US 3 809 714 (Interx; 7.5.1974; prior. 31.8.1972) also racemate resolution.
- b DOS 2 152 058 (Klinge; appl. 19.10.1971).
 |
|
| Clinical data | |
|---|---|
| Trade names | Propine, Pivalephrine |
| Synonyms | Dipivefrin |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a686005 |
| Pregnancy category |
|
| Routes of administration |
Eye drops |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H29NO5 |
| Molar mass | 351.437 g/mol g·mol−1 |
| 3D model (JSmol) | |
//////////дипивефрин , ديبيفيفرين , 地匹福林 , Dipivefrine, antiglaucoma, GENERIC, ジピベフリン















