DNDI-VL-2098
CAS 681492-17-1
(R)-2-Methyl-6-nitro-2-(4-trifluoromethoxyphenoxymethyl)-2,3-dihydroimidazo[2,1-b]oxazole
Watch this post, will be updated………..

(2R)-2-Methyl-6-nitro-2-(4-trifluoromethoxyphenoxymethyl)-2,3-dihydroimidazo[2,1-b]oxazole
Mp: 169–171 °C; Org. Process Res. Dev., Article ASAP, DOI: 10.1021/acs.oprd.6b00331
HPLC (area %): 99.52%; HPLC (chiral): 99.8% (a/a);
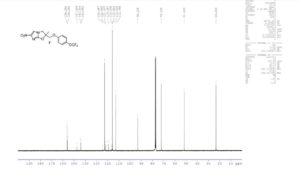
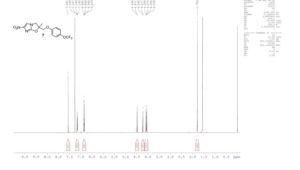
1H NMR (400 MHz, CDCl3): δ 7.57 (s, 1H), 7.14–7.16 (d, 2H, J = 10.0 Hz), 6.83–6.86 (d, 2H, J = 7.2 Hz), 4.48–4.50 (d, 1H, J = 10.0 Hz), 4.22–4.24 (d, 1H, J = 10.0 Hz), 4.05–4.10 (t, 2H, J = 9.6 and 10.4 Hz), 1.79 (s, 3H);
13C NMR (100 MHz, CDCl3): δ 156.0, 155.8, 147.1, 143.5, 122.6, 115.5, 112.6, 122.6, 121.7, and 119.1 (JC–F = 255.1 Hz), 116.6, 92.9, 71.8, 51.3, 23.0;
19F NMR (CDCl3, 376 MHz): δ −58.4;
IR (KBr, cm–1): 3155, 2996, 1607, 1456, 1281, 1106, 978, 921, 834,783, 708;
mass (m/z): 360.3 (M + 1)+;
[α]25589 = (+)8.445 (c 1.00 g/100 mL, CHCl3).
Visceral leishmaniasis (VL), infamously known as kala-azar (black fever) in the Indian subcontinent, is the most lethal form of leishmaniasis and is caused by protozoan parasites. This deadly disease is the second largest parasitic killer in the world, surpassed only by malaria, with a worldwide distribution in Asia, East Africa, South America, and the Mediterranean region. In the search for effective treatments for visceral leishmaniasis, the Drugs for Neglected Diseases initiative (DNDi) recently evaluated fexinidazole a nitroimidazole being developed as a treatment for Human African Trypanosomiasis. Fexinidazole showed potential as a safe and effective oral drug for the treatment of visceral leishmaniasis and is now in clinical trials.

fexinidazole (1) and DNDI-VL-2098 (2).
Earlier, through an agreement with TB Alliance and in association with the ACSRC at the University of Auckland (NZ), DNDi screened about 70 other nitroimidazole analogues belonging to four chemical subclasses and investigated them for antileishmanial activity


Paper
http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.5b01699
Repositioning Antitubercular 6-Nitro-2,3-dihydroimidazo[2,1-b][1,3]oxazoles for Neglected Tropical Diseases: Structure–Activity Studies on a Preclinical Candidate for Visceral Leishmaniasis
Abstract

6-Nitro-2,3-dihydroimidazo[2,1-b][1,3]oxazole derivatives were initially studied for tuberculosis within a backup program for the clinical trial agent pretomanid (PA-824). Phenotypic screening of representative examples against kinetoplastid diseases unexpectedly led to the identification of DNDI-VL-2098 as a potential first-in-class drug candidate for visceral leishmaniasis (VL). Additional work was then conducted to delineate its essential structural features, aiming to improve solubility and safety without compromising activity against VL. While the 4-nitroimidazole portion was specifically required, several modifications to the aryloxy side chain were well-tolerated e.g., exchange of the linking oxygen for nitrogen (or piperazine), biaryl extension, and replacement of phenyl rings by pyridine. Several less lipophilic analogues displayed improved aqueous solubility, particularly at low pH, although stability toward liver microsomes was highly variable. Upon evaluation in a mouse model of acute Leishmania donovani infection, one phenylpyridine derivative (37) stood out, providing efficacy surpassing that of the original preclinical lead.

Structures of various antileishmanial or antitubercular agents.
CLICK ON IMAGE
2-Methyl-6-nitro-2-{[4-(trifluoromethoxy)phenoxy]methyl}-2,3-dihydroimidazo[2,1- b][1,3]oxazole (7).
Method A (Scheme 1B): Reaction of alcohol 88 with NaH, using procedure C, followed by chromatography of the product on silica gel, eluting with CH2Cl2, gave 71 (87%) as a pale yellow solid: mp (CH2Cl2/hexane) 122-124 C (lit.1 mp 126.8-127.9 C); 1 H NMR (CDCl3) 7.56 (s, 1 H), 7.16 (br d, J = 9.1 Hz, 2 H), 6.85 (br d, J = 9.2 Hz, 2 H), 4.48 (d, J = 10.2 Hz, 1 H), 4.23 (d, J = 10.1 Hz, 1 H), 4.09 (d, J = 10.1 Hz, 1 H), 4.05 (d, J = 10.2 Hz, 1 H), 1.79 (s, 3 H); 13C NMR (CDCl3) 156.3 (C-1’), 156.1 (C-7a), 147.4 (C- 6), 143.9 (q, JC-F = 2.1 Hz, C-4’), 122.8 (2 C, C-3’,5’), 120.7 (q, JC-F = 256.5 Hz, 4’-OCF3), 115.8 (2 C, C-2’,6’), 112.8 (C-5), 93.1 (C-2), 72.2 (2-CH2O), 51.6 (C-3), 23.3 (2-CH3). Anal. (C14H12F3N3O5) C, H, N.
Method B (Scheme 2B): Reaction of 2-bromo-1-[(2-methyloxiran-2-yl)methyl]-4-nitro-1Himidazole2 (98) with 4-(trifluoromethoxy)phenol (0.95 equiv) and NaH (1.2 equiv), using procedure I, followed by chromatography of the product on silica gel, eluting with 2:1 and 3:1 CH2Cl2/petroleum ether (foreruns) and then with 3:1 CH2Cl2/petroleum ether and CH2Cl2, S8 gave a crude product, which was crystallized from CH2Cl2/hexane (and the mother liquors further purified by chromatography on silica gel, eluting as before), to give 71 (55%) as a pale yellow solid (see data above). Method C (Scheme 2D): Reaction of 2-chloro-1-[(2-methyloxiran-2-yl)methyl]-4-nitro-1Himidazole1 (109) with 4-(trifluoromethoxy)phenol (1.0 equiv) and NaH, using procedure I, followed by chromatography of the product on silica gel, eluting with 1:1 and 3:2 CH2Cl2/petroleum ether (foreruns) and then with 3:1 CH2Cl2/petroleum ether and CH2Cl2, gave a crude product, which was crystallized from CH2Cl2/hexane (and the mother liquors further purified by chromatography on silica gel, eluting with 1:1 and 3:1 Et2O/petroleum ether and then with Et2O and CH2Cl2), to give 71 (51%) as a pale yellow solid (see data above).
Synthesis of 9 (Scheme 2A): (2R)-2-Methyl-6-nitro-2-{[4-(trifluoromethoxy)phenoxy]methyl}-2,3-dihydroimidazo- [2,1-b][1,3]oxazole (9). Reaction of 2-chloro-1-{[(2R)-2-methyloxiran-2-yl]methyl}-4-nitro- 1H-imidazole3 (96) with 4-(trifluoromethoxy)phenol and NaH, using procedure H, gave 91,3 (36%) as a pale brown solid: mp 170-171 C (lit.1 mp 176.5-178 C); 1 H NMR (CDCl3) 7.56 (s, 1 H), 7.16 (br d, J = 8.8 Hz, 2 H), 6.85 (br d, J = 9.0 Hz, 2 H), 4.48 (d, J = 10.2 Hz, 1 H), 4.23 (d, J = 10.0 Hz, 1 H), 4.09 (d, J = 10.2 Hz, 1 H), 4.05 (d, J = 10.3 Hz, 1 H), 1.79 (s, 3 H); [α] 25 D 9.0 (c 1.002, CHCl3) [lit.1 [α] 28 D 7.67 (c 1.030, CHCl3)]. Anal. (C14H12F3N3O5) C, H, N. HPLC purity: 100%. Chiral HPLC (using a CHIRALPAK AD-H analytical column and eluting with 15% EtOH/hexane at 1 mL/min) determined that the ee of 9 was 98.7%.
Paper
Sasaki, Hirofumi; Journal of Medicinal Chemistry 2006, VOL 49(26), Pg 7854-7860
Synthesis and Antituberculosis Activity of a Novel Series of Optically Active 6-Nitro-2,3-dihydroimidazo[2,1-b]oxazoles
Abstract

In an effort to develop potent new antituberculosis agents that would be effective against both drug-susceptible and drug-resistant strains of Mycobacterium tuberculosis, we prepared a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b]oxazoles substituted at the 2-position with various phenoxymethyl groups and a methyl group and investigated the in vitro and in vivo activity of these compounds. Several of these derivatives showed potent in vitro and in vivo activity, and compound 19 (OPC-67683) in particular displayed excellent in vitro activity against both drug-susceptible and drug-resistant strains of M. tuberculosis H37Rv (MIC = 0.006 μg/mL) and dose-dependent and significant in vivo efficacy at lower oral doses than rifampicin in mouse models infected with M. tuberculosis Kurono. The synthesis and structure−activity relationships of these new compounds are presented.
(R)-2-Methyl-6-nitro-2-(4-trifluoromethoxyphenoxymethyl)-2,3-dihydroimidazo[2,1-b]oxazole (8). Mp 176−178 °C.
1H NMR (CDCl3) δ 1.79 (3H, s), 4.06 (1H, d, J = 6.8 Hz), 4.10 (1H, d, J = 6.8 Hz), 4.23 (1H, d, J = 10.1 Hz), 4.49 (1H, d, J = 10.1 Hz), 6.84 (2H, d, J = 9.0 Hz), 7.13 (2H, d, J = 9.0 Hz), 7.56 (1H, s).
MS (DI) m/z 359 (M+). Anal. (C14H12F3N3O5) C, H, N.
PAPER

A process suitable for kilogram-scale synthesis of (2R)-2-methyl-6-nitro-2-{[4-(trifluoromethoxy)phenoxy]methyl}-2,3-dihydroimidazo[2,1-b][1,3]oxazole (DNDI-VL-2098, 2), a preclinical drug candidate for the treatment of visceral leishmaniasis, is described. The four-step synthesis of the target compound involves the Sharpless asymmetric epoxidation of 2-methyl-2-propen-1-ol, 8. Identification of a suitable synthetic route using retrosynthetic analysis and development of a scalable process to access several kilograms of 2 are illustrated. The process was simplified by employing in situ synthesis of some intermediates, reducing safety hazards, and eliminating the need for column chromatography. The improved reactions were carried out on the kilogram scale to produce 2 in good yield, high optical purity, and high quality.
http://pubs.acs.org/doi/abs/10.1021/acs.oprd.6b00331