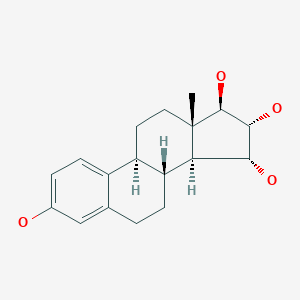
Estetrol
エステトロール;
| Formula |
C18H24O4
|
|---|---|
| CAS |
15183-37-6
|
| Mol weight |
304.3808
|
FDA 4/15/2021, To prevent pregnancy, Nextstellis
New Drug Application (NDA): 214154
Company: MAYNE PHARMA
Label (PDF)
Letter (PDF)
Review
PATENT
https://patents.google.com/patent/EP1562976B1/en
Estrogenic substances are commonly used in methods of Hormone Replacement Therapy (HRT) and methods of female contraception. These estrogenic substances can be divided in natural estrogens and synthetic estrogens. Examples of natural estrogens that have found pharmaceutical application include estradiol, estrone, estriol and conjugated equine estrogens. Examples of synthetic estrogens, which offer the advantage of high oral bioavailability include ethinyl estradiol and mestranol.
Fishman J., Guzik H., J. Org. Chem. 33, 3133 – 3135 (1968) discloses a successful synthesis of estetrol from an estrone derivative (compound (III); cf. for a synthesis of compound (III) Cantrall, E.W., Littell, R., Bernstein, S. J. Org. Chem 29, 214 – 217 (1964)). In a first step, the carbonyl group at C17 of compound (III) was reduced with LiAlH4 to estra-1,3,5(10),15-tetraene-3,17-diol (compound VIa) that was isolated as the diacetate (compound VIb). Compound VIb was subjected to cis-hydroxylation of the double bond of ring D by using OsO4 which resulted into the formation of estra-1,3,5(10)-triene-3,15α,16α,17β-tetraol-3,17-diacetate (compound Ib) that under heating with K2CO3 in methanol produces estetrol (Scheme 1).
The overall yield of this three step process is, starting from estrone derivative III, only about 7%. It is worth noting that the protected derivative 17,17-ethylenedioxyestra-1,3,5(10),15-tetraene-3-ol-3-acetate (compound IV) could be cis-hydroxylated to its 15α,16α-diol derivative (compound Va), but that thereafter the dioxolane group could not be removed (p-toluene sulfonic acid in acetone at room temperature) or that the hydrolysis (aqueous sulfuric acid in warm dioxane) of the dioxolane group resulted in a mixture containing a multitude of products (Scheme 2).
Suzuki E. et al., Steroids 60, 277 – 284 (1995) also discloses the synthesis of estetrol by using compound Vb of Nambara T. et al. as starting material. The carbonyl group at C17 of this compound was first reduced followed by acetylation yielding estra-1,3,5(10),15-tetraene-3,17-diol-3,17-diacetate (compound 2b). The latter was subjected to oxidation with OsO4 which provided estra-1,3,5(10)-triene-3,15α,16α,17β-tetraol-3,17-diacetate (compound 3b) in 46% yield.
Poirier D., et al., Tetrahedron 47, 7751 – 7766 (1991) discloses the following compounds which were prepared according to methods that have been used to prepare similar compounds:
Example 7 3-Benzyloxy-estra-1,3,5 (10),15-tetraen-17-ol (compound 5; A = benzyl)
[0088]
Example 8 17-Acetyloxy-3-benzyloxy-estra-1,3,5 (10),15-tetraene (compound 4; A = benzyl, C = acetyl)
[0089]
Example 9 17-Acetyl-3-Benzyl estetrol (compound 3; A = benzyl, C = acetyl)
[0090]
Example 10 17-Acetyl estetrol (compound 2; C = acetyl)
[0091]
Example 11 Estetrol
[0092]
SYN
https://www.tandfonline.com/doi/abs/10.1080/13697130802054078?journalCode=icmt20
Estetrol (E4), or oestetrol, is a weak estrogen steroid hormone, which is found in detectable levels only during pregnancy in humans.[1][2] It is produced exclusively by the fetal liver.[1] Estetrol is closely related to estriol (E3), which is also a weak estrogen that is found in high quantities only during pregnancy.[1][2] Along with estradiol (E2), estrone (E1), and E3, estetrol (E4) is a major estrogen in the body, although only during pregnancy.[1]
In addition to its role as a natural hormone, estetrol is under clinical development for use as a medication, for instance in hormonal contraception (in combination with drospirenone) and as menopausal hormone therapy; for information on estetrol as a medication, see the estetrol (medication) article.
Biological function
Estetrol is an estrogen and has estrogenic effects in various tissues.[1] Estetrol interacts with nuclear Estrogen Receptor (ERα) in a manner identical to that of the other estrogens and distinct from that observed with Selective Estrogen Receptor Modulators (SERMs).[3][4] So far the physiological function of estetrol is unknown. The possible use of estetrol as a marker for fetal well-being has been studied quite extensively. However, due to the large intra- and inter-individual variation of maternal estetrol plasma levels during pregnancy this appeared not to be feasible.[5][6][7][8][9]
Biological activity
Estetrol is an agonist of the estrogen receptors (ERs), and hence is an estrogen.[10][11] It has moderate affinity for ERα and ERβ, with Ki values of 4.9 nM and 19 nM, respectively.[10][12] As such, estetrol has 4- to 5-fold preference for the ERα over the ERβ.[10][12] The estrogen has low affinity for the ERs relative to estradiol, and both estetrol and the related estrogen estriol require substantially higher concentrations than estradiol to produce similar effects to estradiol.[10] The affinity of estetrol for the ERs is about 0.3% (rat) to 6.25% (human) of that of estradiol, and its in vivo potency in animals is about 2 to 3% of that of estradiol.[10] Estetrol shows high selectivity for the ERs.[10][12]
Biochemistry
Biosynthesis
Estetrol is synthesized during pregnancy only in the fetal liver from estradiol (E2) and estriol (E3) by the two enzymes 15α- and 16α-hydroxylase.[13][14][15] Alternatively, estetrol is synthesized with 15α-hydroxylation of 16α-hydroxy-DHEA sulfate as an intermediate step.[16] It appears in maternal urine at around week 9 of pregnancy.[2] After birth the neonatal liver rapidly loses its capacity to synthesize estetrol because these two enzymes are no longer expressed.
Estetrol reaches the maternal circulation through the placenta and was already detected at nine weeks of pregnancy in maternal urine.[17][18] During the second trimester of pregnancy high levels were found in maternal plasma, with steadily rising concentrations of unconjugated estetrol to about 1 ng/mL (>3 nM) towards the end of pregnancy.[1]
Distribution
In terms of plasma protein binding, estetrol is moderately bound to albumin, and is not bound to sex hormone-binding globulin (SHBG).[19][20]
Metabolism
Estetrol undergoes no phase I metabolism by CYP P450 enzymes.[10] It is conjugated via glucuronidation and to a lesser extent sulfation and then excreted.[10][21]
Excretion
Estetrol is excreted mostly or completely in urine.[21][10]
Chemistry
Estetrol, also known as 15α-hydroxyestriol or as estra-1,3,5(10)-triene-3,15α,16α,17β-tetrol, is a naturally occurring estrane steroid and derivative of estrin (estratriene).[10][11] It has four hydroxyl groups, which explains the abbreviation E4.[10][11]
Synthesis
Chemical syntheses of estetrol have been published.[22]
History
Estetrol was discovered in 1965 by Egon Diczfalusy and coworkers at the Karolinska Institute in Stockholm, Sweden, via isolation from the urine of pregnant women.[10][23]
References
- ^ Jump up to:a b c d e f Holinka CF, Diczfalusy E, Coelingh Bennink HJ (May 2008). “Estetrol: a unique steroid in human pregnancy”. J. Steroid Biochem. Mol. Biol. 110 (1–2): 138–43. doi:10.1016/j.jsbmb.2008.03.027. PMID 18462934.
- ^ Jump up to:a b c Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, 3rd ed., SSC Yen and RB Jaffe (eds.), pp. 936–981, Copyright Elsevier/Saunders 1991
- ^ Abot, Anne; Fontaine, Coralie; Buscato, Mélissa; Solinhac, Romain; Flouriot, Gilles; Fabre, Aurélie; Drougard, Anne; Rajan, Shyamala; Laine, Muriel; Milon, Alain; Muller, Isabelle (2014). “The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor α modulation, uncoupling nuclear and membrane activation”. EMBO Molecular Medicine. 6 (10): 1328–1346. doi:10.15252/emmm.201404112. ISSN 1757-4676. PMC 4287935. PMID 25214462.
- ^ Foidart, JM; et al. (2019). “30th Annual Meeting of The North America Menopause Society September 25 – 28, 2019, Chicago, IL”. Menopause. 26 (12): 1445–1481. doi:10.1097/GME.0000000000001456. ISSN 1530-0374.
- ^ J. Heikkilä, T. Luukkainen, Urinary excretion of estriol and 15a-hydroxyestriol in complicated pregnancies, Am. J. Obstet. Gynecol. 110 (1971) 509-521.
- ^ D. Tulchinsky, F.D. Frigoletto, K.J. Ryan, J. Fishman, Plasma estetrol as an index of fetal well-being, J. Clin. Endocrinol. Metab. 40 (1975) 560-567
- ^ A.D. Notation, G.E. Tagatz, Unconjugated estriol and 15a-hydroxyestriol in complicated pregnancies, Am. J. Obstet. Gynecol. 128 (1977) 747-756.
- ^ N. Kundu, M. Grant, Radioimmunoassay of 15a-hydroxyestriol (estetrol) in pregnancy serum, Steroids 27 (1976) 785-796.
- ^ N. Kundu, M. Wachs, G.B. Iverson, L.P. Petersen, Comparison of serum unconjugated estriol and estetrol in normal and complicated pregnancies, Obstet. Gynecol. 58 (1981) 276-281.
- ^ Jump up to:a b c d e f g h i j k l Coelingh Bennink HJ, Holinka CF, Diczfalusy E (2008). “Estetrol review: profile and potential clinical applications”. Climacteric. 11 Suppl 1: 47–58. doi:10.1080/13697130802073425. PMID 18464023.
- ^ Jump up to:a b c Visser M, Coelingh Bennink HJ (March 2009). “Clinical applications for estetrol” (PDF). J. Steroid Biochem. Mol. Biol. 114(1–2): 85–9. doi:10.1016/j.jsbmb.2008.12.013. PMID 19167495.
- ^ Jump up to:a b c Visser M, Foidart JM, Coelingh Bennink HJ (2008). “In vitro effects of estetrol on receptor binding, drug targets and human liver cell metabolism”. Climacteric. 11 Suppl 1: 64–8. doi:10.1080/13697130802050340. PMID 18464025.
- ^ J. Schwers, G. Eriksson, N. Wiqvist, E. Diczfalusy, 15a-hydroxylation: A new pathway of estrogen metabolism in the human fetus and newborn, Biochim. Biophys. Acta. 100 (1965) 313-316
- ^ J. Schwers, M. Govaerts-Videtsky, N. Wiqvist, E. Diczfalusy, Metabolism of oestrone sulphate by the previable human foetus, Acta Endocrinol. 50 (1965) 597-610.
- ^ S. Mancuso, G. Benagiano, S. Dell’Acqua, M. Shapiro, N. Wiqvist, E. Diczfalusy, Studies on the metabolism of C-19 steroids in the human foeto-placental unit, Acta Endocrinol. 57 (1968) 208-227.
- ^ Jerome Frank Strauss; Robert L. Barbieri (2009). Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 262–. ISBN 1-4160-4907-X.
- ^ J. Heikkilä, H. Adlercreutz, A method for the determination of urinary 15α-hydroxyestriol and estriol, J. Steroid Biochem. 1 (1970) 243-253
- ^ J. Heikkilä, Excretion of 15α-hydroxyestriol and estriol in maternal urine during normal pregnancy, J. Steroid Biochem. 2 (1971) 83-93.
- ^ Visser M, Holinka CF, Coelingh Bennink HJ (2008). “First human exposure to exogenous single-dose oral estetrol in early postmenopausal women”. Climacteric. 11 Suppl 1: 31–40. doi:10.1080/13697130802056511. PMID 18464021.
- ^ Hammond GL, Hogeveen KN, Visser M, Coelingh Bennink HJ (2008). “Estetrol does not bind sex hormone binding globulin or increase its production by human HepG2 cells”. Climacteric. 11 Suppl 1: 41–6. doi:10.1080/13697130701851814. PMID 18464022.
- ^ Jump up to:a b Mawet M, Maillard C, Klipping C, Zimmerman Y, Foidart JM, Coelingh Bennink HJ (2015). “Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives”. Eur J Contracept Reprod Health Care. 20 (6): 463–75. doi:10.3109/13625187.2015.1068934. PMC 4699469. PMID 26212489.
- ^ Warmerdam EG, Visser M, Coelingh Bennink HJ, Groen M (2008). “A new route of synthesis of estetrol”. Climacteric. 11 Suppl 1: 59–63. doi:10.1080/13697130802054078. PMID 18464024.
- ^ Hagen AA, Barr M, Diczfalusy E (June 1965). “Metabolism of 17-beta-oestradiol-4-14-C in early infancy”. Acta Endocrinol. 49: 207–20. doi:10.1530/acta.0.0490207. PMID 14303250.
 |
|
 |
|
| Names | |
|---|---|
| Preferred IUPAC name
(1R,2R,3R,3aS,3bR,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,2,3,7-tetrol
|
|
| Other names
Oestetrol; E4; 15α-Hydroxyestriol; Estra-1,3,5(10)-triene-3,15α,16α,17β-tetrol
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ECHA InfoCard | 100.276.707 |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C18H24O4 | |
| Molar mass | 304.386 g/mol |
| 1.38 mg/mL | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
//////////estetrol, Nextstellis, fda 2021, approvals 2021