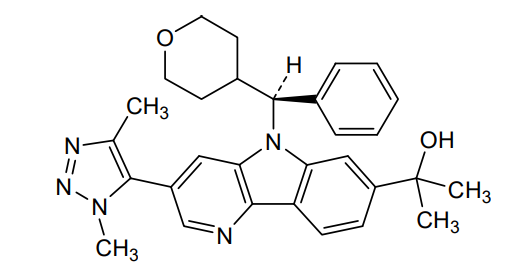
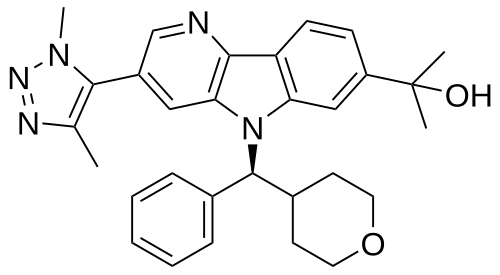
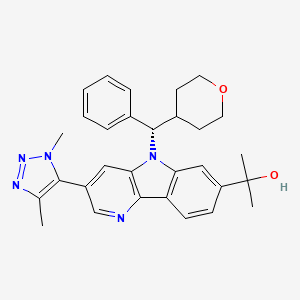
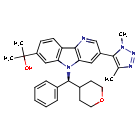
Ezobresib
CAS 1800340-40-2
MF C30H33N5O2 MW 495.6 g/mol
2-{3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5-[(S)-(oxan-4-yl)(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl}propan-2-ol
bromodomain and extra-terminal motif (BET) inhibitor,
antineoplastic, BMS-986158, BMS 986158, Bristol Myers Squibb, antineoplastic, UNII-X8BW0MQ5PI
2-[3-(3,5-dimethyltriazol-4-yl)-5-[(S)-oxan-4-yl(phenyl)methyl]pyrido[3,2-b]indol-7-yl]propan-2-ol
Ezobresib is an investigational new drug that has been evaluated for the treatment of cancer. It inhibits Bromodomain and Extra-Terminal domain (BET) proteins, with potential antineoplastic activity.[1] Developed by Bristol Myers Squibb, this therapeutic agent has been studied for its efficacy in treating various cancers, including solid tumors and hematological malignancies.[2] Despite showing promise in early-phase clinical trials, recent developments suggest that Bristol Myers Squibb has decided to discontinue further development of ezobresib.[3]
BMS-986158 is under investigation in clinical trial NCT02419417 (Study of BMS-986158 in Subjects With Select Advanced Cancers).
Ezobresib is an inhibitor of the Bromodomain (BRD) and Extra-Terminal domain (BET) family of proteins, with potential antineoplastic activity. Upon administration, ezobresib binds to the acetyl-lysine binding site in the BRD of BET proteins, thereby preventing the interaction between BET proteins and acetylated histones. This disrupts chromatin remodeling and prevents the expression of certain growth-promoting genes, resulting in an inhibition of tumor cell growth. BET proteins (BRD2, BRD3, BRD4 and BRDT) are transcriptional regulators that bind to acetylated lysines on the tails of histones H3 and H4, and regulate chromatin structure and function; they play an important role in the modulation of gene expression during development and cellular growth
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US206490064&_cid=P21-MGLNPO-16484-1
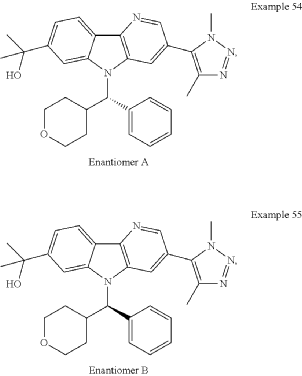
Examples 54 & 55
2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-5-[oxan-4-yl(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl]propan-2-ol
Step 1: 2-Chloro-5-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)pyridin-3-amine
Step 2: Methyl 3-((2-chloro-5-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)pyridin-3-yl)amino)benzoate
Step 3: Methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5H-pyrido[3,2-b]indole-7-carboxylate
Alternate synthesis of Methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5H-pyrido[3,2-b]indole-7-carboxylate
Step 4: Methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5-(phenyl(tetrahydro-2H-pyran-4-yl)methyl)-5H-pyrido[3,2-b]indole-7-carboxylate
Step 5: 2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-5-[oxan-4-yl(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl]propan-2-ol
Alternate Synthesis of Examples 54
2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-5-[oxan-4-yl(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl]propan-2-ol
Step 1: 2-Chloro-5-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)pyridin-3-amine
Step 2: Methyl 3-((2-chloro-5-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)pyridin-3-yl)amino)benzoate
Step 3: Methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5H-pyrido[3,2-b]indole-7-carboxylate
Alternate synthesis of Methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5H-pyrido[3,2-b]indole-7-carboxylate
Step 4: Methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5-(phenyl(tetrahydro-2H-pyran-4-yl)methyl)-5H-pyrido[3,2-b]indole-7-carboxylate
Step 5: 2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-5-[oxan-4-yl(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl]propan-2-ol
Alternate Synthesis of Examples 54
2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-5-[oxan-4-yl(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl]propan-2-ol
Step 1: (S)-methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5-(phenyl(tetrahydro-2H-pyran-4-yl)methyl)-5H-pyrido[3,2-b]indole-7-carboxylate
Step 2. (S)-2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-5-[oxan-4-yl(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl]propan-2-ol
Step 1: (S)-methyl 3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5-(phenyl(tetrahydro-2H-pyran-4-yl)methyl)-5H-pyrido[3,2-b]indole-7-carboxylate
Step 2. (S)-2-[3-(Dimethyl-1H-1,2,3-triazol-5-yl)-5-[oxan-4-yl(phenyl)methyl]-5H-pyrido[3,2-b]indol-7-yl]propan-2-ol
PATENT
LIT
- BLM overexpression as a predictive biomarker for CHK1 inhibitor response in PARP inhibitor–resistant BRCA -mutant ovarian cancerPublication Name: Science Translational MedicinePublication Date: 2023-06-21PMCID: PMC10758289PMID: 37343085DOI: 10.1126/scitranslmed.add7872
- Recent updates on 1,2,3-triazole-containing hybrids with in vivo therapeutic potential against cancers: A mini-reviewPublication Name: European Journal of Medicinal ChemistryPublication Date: 2023-05-05PMID: 36893627DOI: 10.1016/j.ejmech.2023.115254
- Development of BET Inhibitors as Potential Treatments for Cancer: Optimization of Pharmacokinetic PropertiesPublication Name: ACS Medicinal Chemistry LettersPublication Date: 2022-07-05PMCID: PMC9290009PMID: 35859878DOI: 10.1021/acsmedchemlett.2c00219
- Synthesis of BMS-986158Publication Name: SynfactsPublication Date: 2021-11-17DOI: 10.1055/s-0041-1737090
- Development of BET inhibitors as potential treatments for cancer: A new carboline chemotypePublication Name: Bioorganic & Medicinal Chemistry LettersPublication Date: 2021-11-01PMID: 34560263DOI: 10.1016/j.bmcl.2021.128376
- Discovery and Preclinical Pharmacology of an Oral Bromodomain and Extra-Terminal (BET) Inhibitor Using Scaffold-Hopping and Structure-Guided Drug DesignPublication Name: Journal of Medicinal ChemistryPublication Date: 2021-09-20PMID: 34543572DOI: 10.1021/acs.jmedchem.1c00625
- Retrospective assessment of rat liver microsomal stability at NCATS: data and QSAR modelsPublication Name: Scientific ReportsPublication Date: 2020-11-26PMCID: PMC7693334PMID: 33244000DOI: 10.1038/s41598-020-77327-0
- High-Throughput Screening to Identify Inhibitors of the Type I Interferon–Major Histocompatibility Complex Class I Pathway in Skeletal MusclePublication Name: ACS Chemical BiologyPublication Date: 2020-05-27PMCID: PMC7859889PMID: 32459468DOI: 10.1021/acschembio.0c00343
- Predictive models of aqueous solubility of organic compounds built on A large dataset of high integrityPublication Name: Bioorganic & Medicinal ChemistryPublication Date: 2019-07-15PMCID: PMC8274818PMID: 31176566DOI: 10.1016/j.bmc.2019.05.037
- Highly predictive and interpretable models for PAMPA permeabilityPublication Name: Bioorganic & Medicinal ChemistryPublication Date: 2017-02-01PMCID: PMC5291813PMID: 28082071DOI: 10.1016/j.bmc.2016.12.049
- BET inhibitor resistance emerges from leukaemia stem cellsPublication Name: NaturePublication Date: 2015-09-14PMCID: PMC6069604PMID: 26367796DOI: 10.1038/nature14888
- Efficacy of BET Bromodomain Inhibition in Kras-Mutant Non–Small Cell Lung CancerPublication Name: Clinical cancer research : an official journal of the American Association for Cancer ResearchPublication Date: 2013-11-14PMCID: PMC3838895PMID: 24045185DOI: 10.1158/1078-0432.ccr-12-3904
- Discovery and Preclinical Evaluation of [4-[[1-(3-fluorophenyl)methyl]-1H-indazol-5-ylamino]-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yl]carbamic Acid, (3S)-3-Morpholinylmethyl Ester (BMS-599626), a Selective and Orally Efficacious Inhibitor of Human Epidermal Growth Factor Receptor 1 and 2 KinasesPublication Name: Journal of Medicinal ChemistryPublication Date: 2009-10-12PMID: 19821562DOI: 10.1021/jm9010065
- [Statistical analysis of cerebrospinal fluid acid-base equilibrium and cerebrospinal fluid lactate concentration in cases of brain tumors, cerebrocranial injuries and meningoencephalitis]Publication Name: Neurologia i neurochirurgia polskaPublication Date: 1976-07PMID: 8740
PAT
- Tricyclic compounds as anticancer agentsPublication Number: WO-2015100282-A1Priority Date: 2013-12-24
- Tricyclic compound as anticancer agentsPublication Number: EP-3466949-B1Priority Date: 2013-12-24Grant Date: 2020-12-23
- Novel tricyclic compounds as anticancer agentsPublication Number: TW-202028203-APriority Date: 2013-12-24
- Novel tricyclic compounds as anticancer agentsPublication Number: TW-I726544-BPriority Date: 2013-12-24Grant Date: 2021-05-01
- Tricyclic compounds as anticancer agentsPublication Number: CN-108558871-BPriority Date: 2013-12-24Grant Date: 2022-02-18



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | BMS-986158 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1800340-40-2 |
| PubChem CID | 118196485 |
| DrugBank | DB15435 |
| ChemSpider | 58828664 |
| UNII | X8BW0MQ5PI |
| KEGG | D12710 |
| ChEMBL | ChEMBL4297458 |
| Chemical and physical data | |
| Formula | C30H33N5O2 |
| Molar mass | 495.627 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- Ma Z, Zhang C, Bolinger AA, Zhou J (October 2024). “An updated patent review of BRD4 degraders”. Expert Opinion on Therapeutic Patents. 34 (10): 929–951. doi:10.1080/13543776.2024.2400166. PMC 11427152. PMID 39219068.
- “Clinical Trials Using Ezobresib”. National Cancer Institute.
- Brown A. “Bristol backs out of BET inhibition”. ApexOnco.
////////////Ezobresib, antineoplastic, BMS-986158, BMS 986158, Bristol Myers Squibb, antineoplastic, UNII-X8BW0MQ5PI














