Ferric derisomaltose
WeightAverage: 562.297
Monoisotopic: 562.117975Chemical FormulaC18H34FeO16
Monover, JAPAN 2022, 2022/3/28
Monoferric (TN);
Monover (TN)
Anti-anemic, Hematinic, Supplement (iron)
CAS 1345510-43-1
デルイソマルトース第二鉄
- NS32
- WHO 9712
-
UNII-AHU547PI9H
| Originator Company |
|
| Active Companies |
|
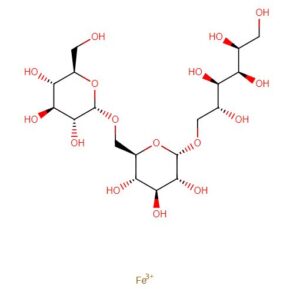
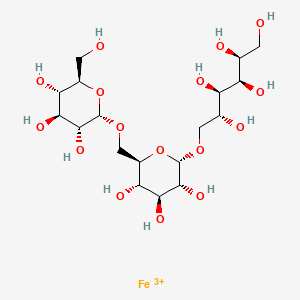
iron(3+) (2S,3R,4R,5R)-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}hexane-1,2,3,4,5-pentol
-
alpha-D-Glucan, (1-6)-, reduced, reaction products with iron hydroxide (Fe(OH)3)
Ferric derisomaltose is an iron injection used in the treatment of iron deficiency anemia.
Ferric derisomaltose, sold under the brand name Monoferric, is a medication for the treatment of iron deficiency anemia (IDA) in adults who have intolerance to oral iron or have had unsatisfactory response to oral iron or who have non-hemodialysis dependent chronic kidney disease (NDD-CKD).[1] It was approved for use in the United States in January 2020.[1][2][3] It is given intravenously.[1]
Iron deficiency is an extremely common condition and is the most frequent cause of anemia worldwide. Iron deficiency results when iron intake, iron stores, and loss of iron from the body do not adequately support production of erythrocytes, also known as red blood cells. Though it is generally considered non life-threatening, iron deficiency may considerably affect quality of life.3
Ferric derisomaltose is a form of iron used in the treatment of iron deficiency. This drug is a complex of iron (III) hydroxide and derisomaltose. The latter is an iron carbohydrate oligosaccharide that works to release iron. Ferric derisomaltose was developed by Pharmacosmos Therapeutics ad was granted FDA approval in January 2020.8,9 Clinical trials show that it is non-inferior to iron sucrose, another form of iron that is often administered in iron deficiency, and less likely to cause serious hypersensitivity that is associated with other forms of injectable iron.1,4
This drug is indicated for the treatment of iron deficiency anemia in adult patients who have experienced intolerance to oral iron preparations or insufficient clinical response to orally administered iron. Ferric derisomaltase is also indicated for patients with non-hemodialysis dependent chronic kidney disease.8 In Australia and United Kingdom, ferric derisomaltase is indicated for cases in which rapid delivery of iron is required.10,11
Iron deficiency is an extremely common condition and is the most frequent cause of anemia worldwide. Iron deficiency results when iron intake, iron stores, and loss of iron from the body do not adequately support production of erythrocytes, also known as red blood cells. Though it is generally considered non life-threatening, iron deficiency may considerably affect quality of life. Ferric derisomaltose is a form of iron used in the treatment of iron deficiency. This drug is a complex of iron (III) hydroxide and derisomaltose. The latter is an iron carbohydrate oligosaccharide that works to release iron. Ferric derisomaltose was developed by Pharmacosmos Therapeutics ad was granted FDA approval in January 2020. Clinical trials show that it is non-inferior to [iron sucrose], another form of iron that is often administered in iron deficiency, and less likely to cause serious hypersensitivity that is associated with other forms of injectable iron.
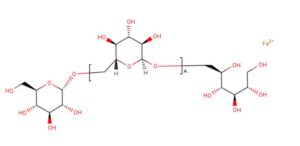
Monoferric is an iron replacement product containing ferric derisomaltose for intravenous infusion. Ferric derisomaltose is an iron carbohydrate complex with a matrix structure composed of interchanging layers of ferric hydroxide and the carbohydrate derisomaltose. Derisomaltose consists of linear, hydrogenated isomaltooligosaccharides with an average molecular weight of 1000 Da and a narrow molecular weight distribution that is almost devoid of mono-and disaccharides.
Ferric derisomaltose has an average molecular weight of 155,000 Da and has the following empirical formula:
{FeO(1-3X) (OH)(1+3X) (C6H5O73-)X}, (H20)T, –
(C6H10O6)R(-C6H10O5-)Z(C6H13O5)R, (NaCl)Y
X= 0.0311; T = 0.25; R = 0.14; Z = 0.49; Y = 0.14
Iron atoms placed in the electronegative cavities of the 3-D structure between and within the derisomaltose molecules. A schematic representation is presented below
 |
Monoferric is a sterile, dark brown, non-transparent aqueous solution with pH 5.0-7.0, containing ferric derisomaltose dissolved in water for injections and filled into Type I glass vials.
Each 1 mL of solution contains 100 mg of elemental iron as ferric derisomaltose in water for injection.
| Mkt. Status |
Active Ingredient | Proprietary Name | Appl. No. | Dosage Form | Route | Strength | TE Code | RLD | RS | Applicant Holder |
|---|---|---|---|---|---|---|---|---|---|---|
| Mkt. Status |
Active Ingredient | Proprietary Name | Appl. No. | Dosage Form | Route | Strength | TE Code | >RLD | RS | Applicant Holder |
| RX | FERRIC DERISOMALTOSE | MONOFERRIC | N208171 | SOLUTION | INTRAVENOUS | 1GM/10ML (100MG/ML) | RLD | RS | PHARMACOSMOS AS | |
| DISCN | FERRIC DERISOMALTOSE | MONOFERRIC | N208171 | SOLUTION | INTRAVENOUS | 100MG/ML (100MG/ML) | RLD | PHARMACOSMOS AS | ||
| DISCN | FERRIC DERISOMALTOSE | MONOFERRIC | N208171 | SOLUTION | INTRAVENOUS | 500MG/5ML (100MG/ML) | RLD | PHARMACOSMOS AS |
MONOFERRIC (FERRIC DERISOMALTOSE)
1GM/10ML (100MG/ML)
Marketing Status: Prescription
PATENT
AU2009342799B2
US10414831B2
US2012010166A1
US2014303364A1
///////////

AS ON DEC2021 3,491,869 VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////
References
- ^ Jump up to:a b c d “Monoferric- ferric derisomaltose solution”. DailyMed. 24 January 2020. Retrieved 16 February 2020.
- ^ “Monoferric approval letter” (PDF). U.S. Food and Drug Administration (FDA). 16 January 2020. Retrieved 16 February 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ “Drug Approval Package: Monoferric Injection”. U.S. Food and Drug Administration (FDA). 7 May 2020. Retrieved 13 August 2020.
External links
- “Ferric derisomaltose”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Trade names | Monoferric |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration |
Intravenous (IV) |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C18H34FeO16+3 |
| Molar mass | 562.299 g·mol−1 |
| 3D model (JSmol) | |
/////////////Ferric derisomaltose, デルイソマルトース第二鉄 , APPROVALS 2022, JAPAN 2022, NS32, WHO 9712
[Fe+3].OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO[C@H]1O[C@H](CO[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@H](O)[C@H]1O