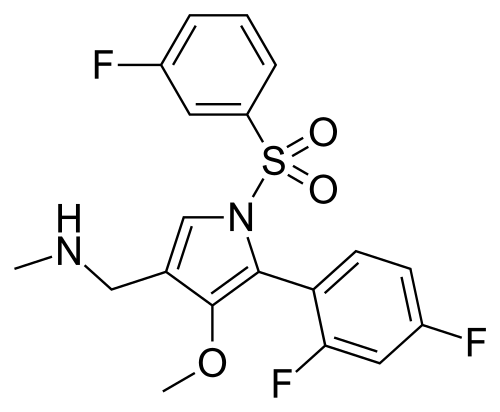
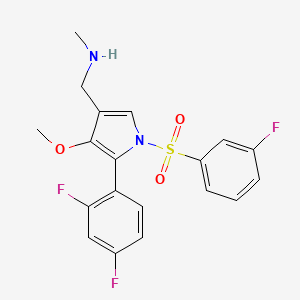
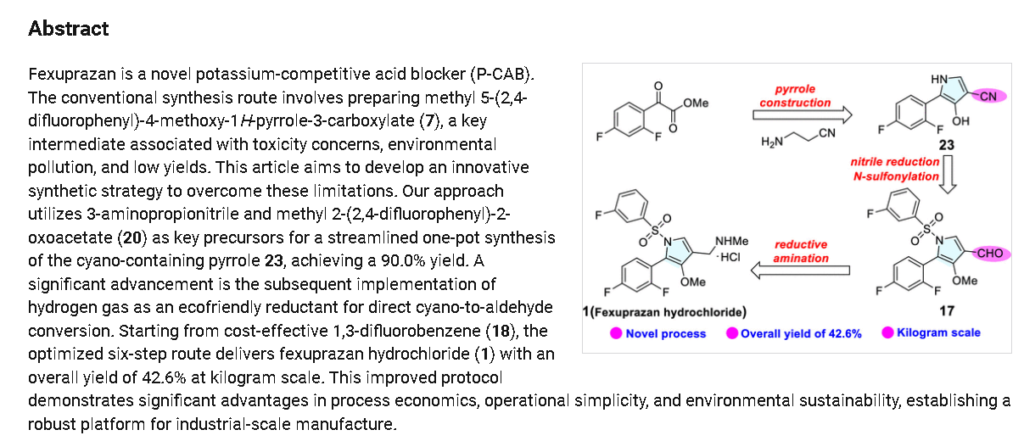
Fexuprazan, Abeprazan; DWP14012; DWP-14012
- CAS 1902954-60-2
- BE52S2C1QT
1-[5-(2,4-difluorophenyl)-1-(3-fluorophenyl)sulfonyl-4-methoxypyrrol-3-yl]-N-methylmethanamine
WeightAverage: 410.41
Monoisotopic: 410.091198078
Chemical FormulaC19H17F3N2O3S
Fexuprazan (trade name Fexuclue) is a drug for the treatment of gastroesophageal reflux disease (GERD).[1] It is a potassium-competitive acid blocker,[2] which is a class of drugs suppressing gastric acids.[3][4]
Fexuprazan is approved for clinical use in South Korea,[4][5] Mexico,[6] Philippines,[7] Chile,[8] and Ecuador.[9]
Abeprazan is under investigation in clinical trial NCT04341454 (Study to Evaluate the Efficacy and Safety of DWP14012 in Patients With Acute or Chronic Gastritis).
Proton pump inhibitors (PPIs) typified by omeprazole, which inhibit gastric acid secretion, are widely used in clinical settings. However, existing PPIs are accompanied by problems in terms of effectiveness and side effects. Specifically, since existing PPIs are unstable under acidic conditions, they are often formulated as enteric agents. in need. In addition, the existing PPI exhibits variation in therapeutic effect due to metabolic enzyme polymorphism and drug interaction with drugs such as diazepam, so improvement is desired.
In addition, since PPI is a prodrug activated by gastric acid and acts only on the active proton pump, the maximum drug expression time is delayed, the effect of suppressing acid secretion at night is poor, and it has disadvantages such as having to take it before meals. exist. In addition, PPI is mainly metabolized through the CYP2C19 enzyme, and there is a large difference in efficacy between individuals due to the genetic polymorphism of the CYP2C19 enzyme.
In order to improve the disadvantages of PPI as described above, a potassium-competitive gastric acid secretion inhibitor (Potassium-Competitive Acid Blocker, P-CAB) is attracting attention. Potassium competitive gastric acid secretion inhibitor strongly and rapidly inhibits gastric acid secretion by reversibly and competitively binding with K + ions to proton pump (H + /K + -ATPase), an enzyme involved in the final stage of gastric acid secretion in parietal cells. These P-CAB formulations show strong inhibition of the normal acidity (pH 1-3) in the stomach compared to the PPI formulations. However, pharmacological activity, which decreases as the pH increases, is required for gastric P-CAB preparations, and some P-CAB preparations show pharmacological activity that maintains pharmacological activity even when the pH increases, and some related side effects have been reported. In addition, since P-CAB preparations are mainly metabolized through the CYP3A4 enzyme, the difference in efficacy between individuals is relatively small, and concerns about interactions with drugs metabolized by the CYP2C19 enzyme are relatively low.
International Patent Publication No. WO2019/013310 A1 discloses bonoprazan as a potassium-competitive acid secretion inhibitor.
However, it was confirmed that vonoprazan induces severe hypergastrinemia compared to the existing PPI drug lansoprazole. Such hypergastrinemia can include enterochromaffin-like (ECL)-cell hyperplasia; parietal cell hyperplasia; fundic gland polyp; It can cause problems such as bone loss, damaged bone quality, and fractures. In fact, it has been reported that vonoprazan is associated with the development of gastric neuroendocrine tumors in carcinogenicity studies in mice and rats. However, discontinuation of administration of P-CAB or PPI-based drugs such as vonoprazan restores excess gastric acid and causes indigestion, so despite the above problems, drug administration cannot be easily stopped.
On the other hand, PPI is used for the prevention of gastric and duodenal ulcers by administration of nonsteroidal anti-inflammatory drugs (NSAIDs). However, it has been reported that bonoprazan aggravates the damage to the small intestine caused by various types of NSAIDs. For example, NSAID-induced gastrointestinal damage includes edema, erythema, submucosal hemorrhage, erosion, and ulceration. From this point of view, clinically, in the case of vonoprazan, there may be significant limitations in combination with NSAID drugs.
There are two major mechanisms by which drugs such as NSAIDs or alcohol cause damage to the gastrointestinal mucosa: a local irritant effect and a systemic effect. The local irritant effect occurs due to ion-trap and mitochondrial damage, and systemically due to the decrease in prostaglandin and NO (nitric oxide). In addition to mitochondrial damage caused by oxidative stress, damage to vascular endothelial cells causes microcirculation disorders, making the gastrointestinal mucosa very vulnerable to damage and interfering with the mucosal damage recovery mechanism. Due to the combined action of these mechanisms, damage to the mucous membrane of the gastrointestinal tract, ie, gastric ulcer, enteropathy, etc. may occur or be severe.
Accordingly, even considering the effect of bonoprazan in terms of suppressing gastric acid secretion, its use is bound to be very limited due to the above potential problems.
Separately, Helicobacter pylori ( H. pylori ) is known as one of the main causes of gastrointestinal diseases such as chronic gastritis and peptic ulcer and gastric cancer. Although the prevalence of Helicobacter pylori in our country is gradually decreasing, a prevalence of more than 50% is still being reported. In particular, Helicobacter pylori is related to digestive diseases, and the importance of antibacterial treatment agents is increasing day by day. In particular, as reported in several studies, antibacterial treatment of Helicobacter pylori reduces the occurrence of bleeding in peptic ulcer. For this antibacterial therapy, in general, patients take clarithromycin and amoxicillin along with gastric acid inhibitors such as PPI as the first-line treatment. For multi-drug use of PPIs and antibiotics, the risk of drug-drug interactions must be low, and the risk of such interactions can be predicted through in vitro CYP inhibition, CYP/UGT phenotyping, and CYP induction tests.
However, additional or repeated administration of various antibiotics is required up to the second and third treatment, and side effects and resistance have been reported. Therefore, by reducing gastric acidity, the antibacterial effect of antibiotics on Helicobacter pylori (H. pylori ) is enhanced, and long-term dose reduction of gastric acidity, for example, proton-potassium pump inhibitory ability, etc. The need to develop a visible drug is emerging.
In addition, in the case of an oral drug, the bioavailability, which is the rate at which the administered drug enters the systemic circulation and is used in the body, is measured. High bioavailability is one of the essential elements of oral drugs because the higher the bioavailability, the higher the rate and extent to which the active ingredient or part of the drug is absorbed and utilized at the site of action. In general, such bioavailability increases as absorption through the gastrointestinal tract is higher and the degree of first-pass effect is lower. , is affected by the size and shape of the particles, and the surface area of the particles.
It is also important that the concentration of the drug in the target organ, in this case the gastric tissue, is maintained as well as the bioavailability in the circulatory system. Therefore, drug distribution and maintenance to the target organ, gastric tissue, is judged to be an important pharmacokinetic characteristic in P-CAB drug development.
On the other hand, somatostatin, also known as growth hormone-inhibiting hormone (GHIH), is a cyclic peptide expressed in the gastrointestinal tract, pancreas, hypothalamus and central nervous system. It is secreted by D cells of the stomach and pancreas and acts as a paracrine regulator of gastric acid secretion, and suppresses gastric acid secretion by inhibiting gastric G cell gastrin secretion and parietal cell acid secretion. Activation of somatostatin receptors by somatostatin analogs and somatostatin receptor agonists inhibit gastrin secretion, thereby regulating histamine release from ECL cells and inhibiting acid secretion. In actual animal models and hypergastrinemia patients, it has been reported that the somatostatin analogue decreased the total gastric acid secretion by decreasing gastrin secretion and gastric acid response.
Gastric acid suppression by taking drugs such as PPI suppresses somatostatin secretion by D cells and promotes gastrin secretion by G cells by a feedback mechanism to induce hypergastrinemia. Gastrin promotes epithelial cell growth to induce oxyntic cell hyperplasia in the gastric body and increase parietal cell mass. This leads to proliferation of adenoma cells and hyperplasia of ECL cells, which may increase the risk of neuroendocrine tumors. In addition, the frequency of neuroendocrine tumors among tumors occurring in the duodenum is relatively high, and it is known that gastrin secretion is the most common form in neuroendocrine tumors occurring in the duodenum, accounting for approximately 65% of the total. It has been confirmed that the group taking bonoprazan tends to have a higher blood gastrin level than the group taking the existing PPI formulation due to the feedback mechanism of excessive gastric acid suppression. Because hypergastrinemia stimulates intestinal endocrine cells and may increase the risk of neuroendocrine tumors, studies are ongoing regarding the safety of long-term use.
Inhibition of gastrin secretion through somatostatin receptor activation has been reported to inhibit ECL cell hyperproliferation. In fact, synthetic peptide analogues of somatostatin with indications for endocrine diseases such as acromegaly, neuroendocrine tumors (NETs), and digestive system diseases such as upper gastrointestinal bleeding Sandostatin® (octreotide acetate) and Somatuline® Depot (lanreotide) are gastric neuroendocrine It has been reported to inhibit the overgrowth of ECL cells by inhibiting gastrin secretion in tumors.
In addition, there have been reports of anti-inflammatory responses through somatostatin receptor activation. Somatostatin is a type of neuropeptide that suppresses neurogenic inflammation and regulates the secretion of hormones and neurotransmitters. It is known to inhibit neurogenic inflammation and to be involved in nociception. Somatostatin is known to control the secretion of hormones and neurotransmitters to suppress neuronal inflammation and to be involved in nociception. Inflammatory somatostatin inhibits the proliferation of T lymphocytes and granulocytes in addition to controlling the neuroendocrine system. Somatostatin analogs are known to increase the expression of the anti-inflammatory factor IL-10 and inhibit the expression of the pro-inflammatory factors IFN-γ and TNF-α. As a result, the anti-inflammatory role of somatostatin has been mainly reported in studies related to inflammatory bowel disease (IBD). It is known that the level of intestinal somatostatin is reduced in patients with IBD, and it is known that the higher the level of inflammation in the intestine, the lower the level of somatostatin. In fact, it has been reported that the somatostatin analogue octreotide improved the symptoms of IBD in patients and animal models.
REF
- The efficacy and safety of fexuprazan in treating erosive esophagitis: a phase III, randomized, double‐blind, multicenter studyPublication Name: Journal of Gastroenterology and HepatologyPublication Date: 2024-01-22PMID: 38251791DOI: 10.1111/jgh.16471
- Review of the clinical development of fexuprazan for gastroesophageal reflux–related diseasePublication Name: European Journal of Clinical PharmacologyPublication Date: 2023-06-22PMID: 37344679DOI: 10.1007/s00228-023-03521-4
- Efficacy and Safety of Fexuprazan in Patients with Acute or Chronic GastritisPublication Name: Gut and LiverPublication Date: 2023-02-15PMCID: PMC10651377PMID: 36789577DOI: 10.5009/gnl220457
- Randomized controlled trial to evaluate the efficacy and safety of fexuprazan compared with esomeprazole in erosive esophagitisPublication Name: World Journal of GastroenterologyPublication Date: 2022-11-28PMCID: PMC9730436PMID: 36504556DOI: 10.3748/wjg.v28.i44.6294
- Editorial: acid suppression with potassium‐competitive acid blockers—dismissing genotype concerns. Authors’ replyPublication Name: Alimentary Pharmacology & TherapeuticsPublication Date: 2020-12-17PMID: 33333601DOI: 10.1111/apt.16158
- Editorial: acid suppression with potassium‐competitive acid blockers dismissing genotype concernsPublication Name: Alimentary Pharmacology & TherapeuticsPublication Date: 2020-12-17PMID: 33333607DOI: 10.1111/apt.16139
- Pharmacodynamics and pharmacokinetics of DWP14012 (fexuprazan) in healthy subjects with different ethnicitiesPublication Name: Alimentary Pharmacology & TherapeuticsPublication Date: 2020-10-27PMID: 33111337DOI: 10.1111/apt.16131
- Safety, tolerability, pharmacodynamics and pharmacokinetics of <scp>DWP</scp>14012, a novel potassium‐competitive acid blocker, in healthy male subjectsPublication Name: Alimentary Pharmacology & TherapeuticsPublication Date: 2018-06-04PMID: 29863280DOI: 10.1111/apt.14818
PATENTS
SearchSubmit searchSort byPublication Number – A to ZPublication Number – Z to APriority Date – OldestPriority Date – Most RecentGrant Date – OldestGrant Date – Most Recent
- Novel 4-methoxypyrrole derivative or salt thereof and pharmaceutical composition containing the samePublication Number: JP-6244498-B1Priority Date: 2015-04-27Grant Date: 2017-12-06
- Novel 4-methoxy pyrrole derivatives or salts thereof and pharmaceutical composition comprising the samePublication Number: KR-20160127646-APriority Date: 2015-04-27
- Novel 4-methexipirols derivatives or salts thereof and pharmaceutical compositions thereofPublication Number: RU-2663895-C1Priority Date: 2015-04-27Grant Date: 2018-08-13
- 4-methoxy pyrrole derivatives or salts thereof and pharmaceutical composition comprising the samePublication Number: US-10100010-B1Priority Date: 2015-04-27Grant Date: 2018-10-16
- Novel 4-methoxy pyrrole derivatives or salts thereof and pharmaceutical composition comprising the samePublication Number: WO-2016175555-A2Priority Date: 2015-04-27
SYN
https://pubs.acs.org/doi/10.1021/acsomega.4c04507
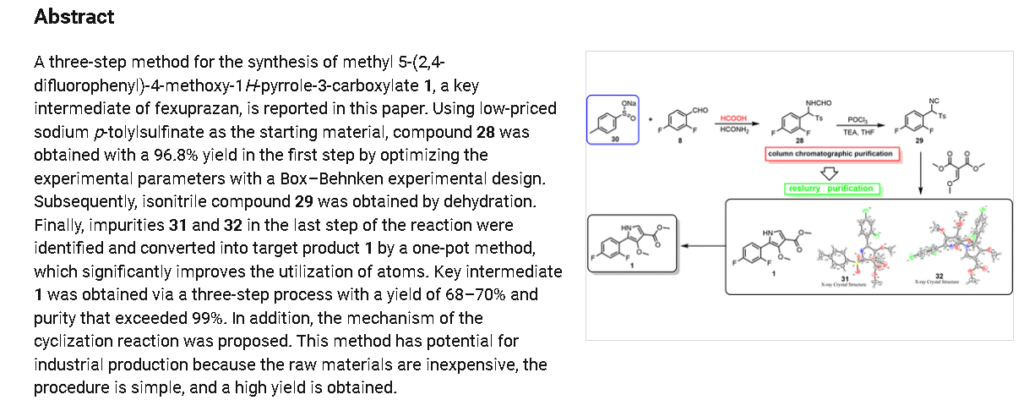
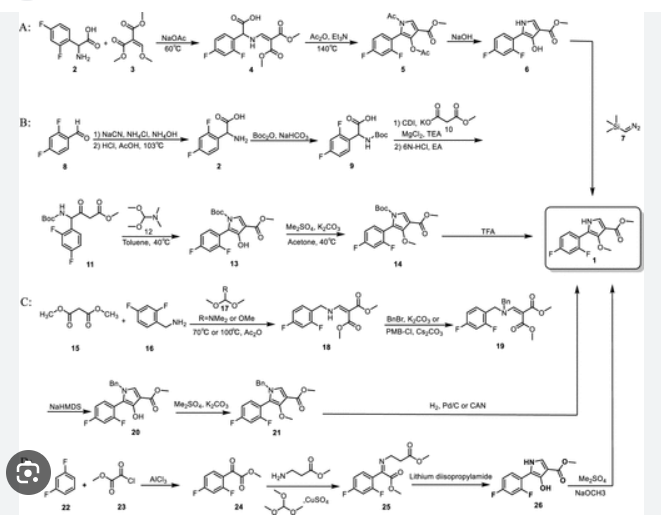
PAT
https://patents.google.com/patent/WO2023211843A1/en
Patent Citations (4)
Publication numberPriority datePublication dateAssigneeTitle
WO2016175555A2 *2015-04-272016-11-03Daewoong Pharmaceutical Co., Ltd.Novel 4-methoxy pyrrole derivatives or salts thereof and pharmaceutical composition comprising the same
US20190031609A1 *2016-03-252019-01-31Daewoong Pharmaceutical Co., Ltd.Novel acid addition salt of 1-(5-(2,4-difluorophenyl)-1-((3-fluorophenyl)sulfonyl)-4-methoxy-1h-pyrrol-3-yl)-n-methylmethanamine
US20200146974A1 *2017-07-072020-05-14Cj Healthcare CorporationComposition for injection
WO2021256861A1 *2020-06-172021-12-23일동제약(주)Novel acid secretion inhibitor and use thereof
PAT
https://patents.google.com/patent/WO2016175555A2/en
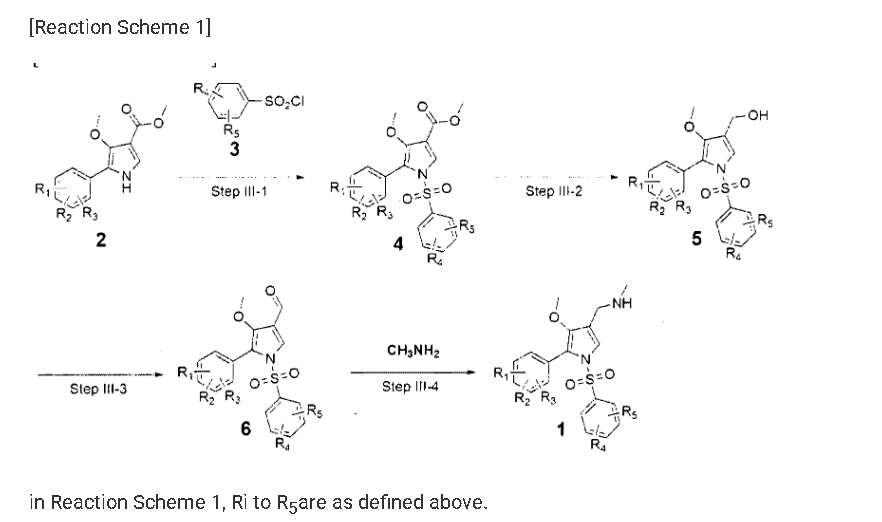
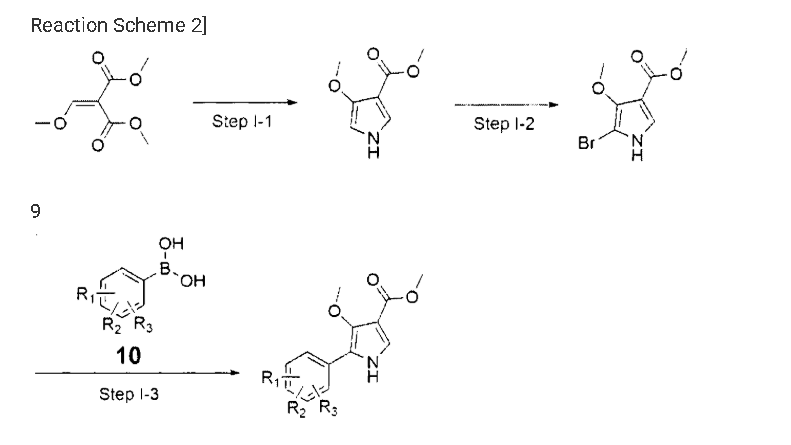
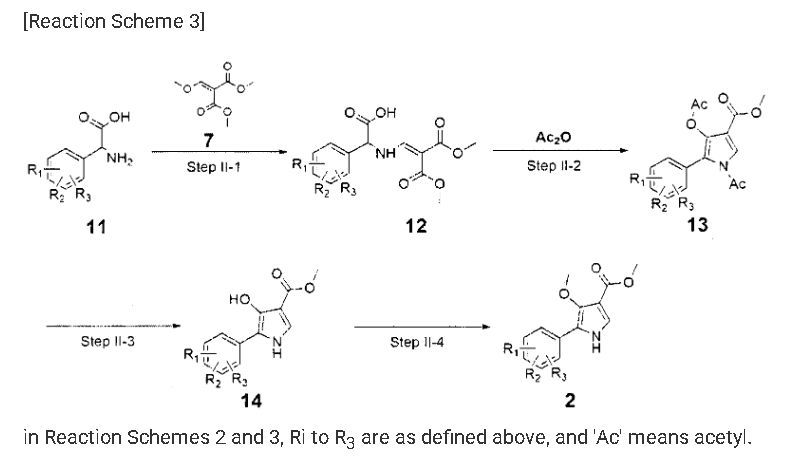
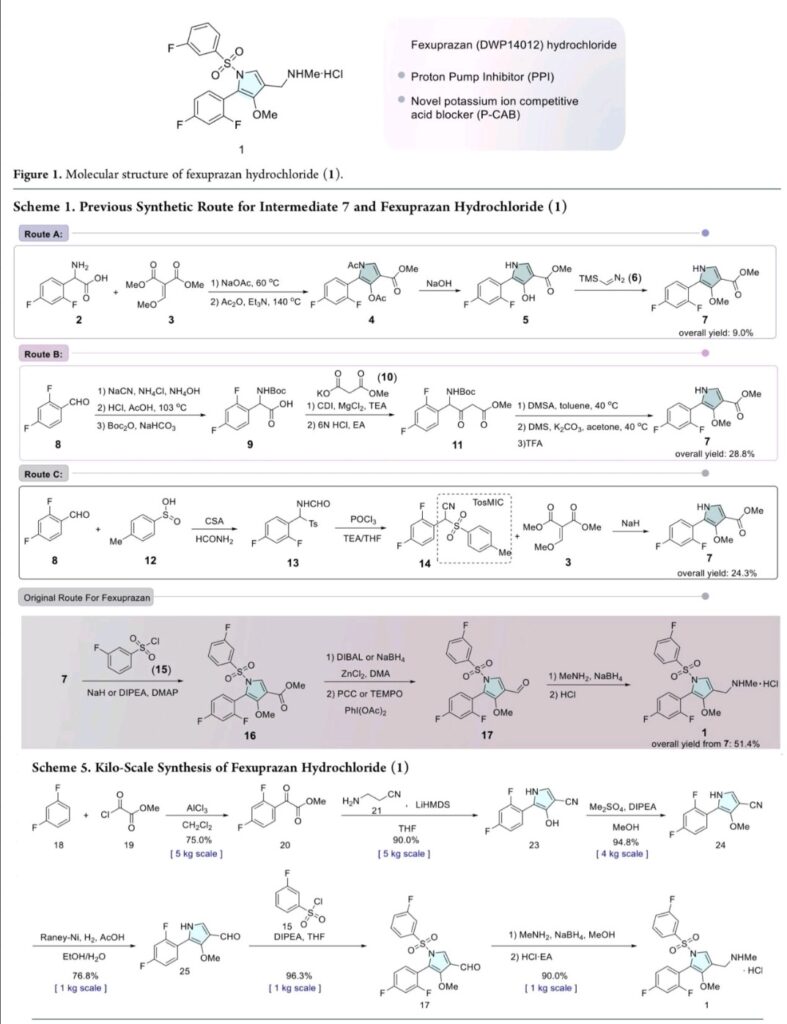
Example 8: Preparation of l-(5-(2,4-difluorophenyI)-l-((3-fluorophenyl)sulfonyI)-
4- metho\ -lH-pyrrol-3-yl)-N-methylmethanamine hydrochloride

(Step 8-1) Preparation of 2-(2,4-difluorophenyl)-2-((3-methoxy-2- (methoxycarbonyl)-3-oxoprop-l-en-l-yl)amino)acetic acid
2,4- Di fluorophenyl glycine (150.0 g, 801.5 mmol), dimethyl 2- (methoxymethylene)malonate (126.9 g, 728.6 mmol), and sodium acetate (65.8 g, 801 .5 mmol) were added to methanol (800.0 ml), and then refJuxed at 60°C for 4 hours. The reaction mixture was cooled to room temperature, and concentrated under reduced pressure to remove about 70% of methanol, and then filtered. The resulting solid was dried reduced pressure to give 190.0 g of the title compound. (Yield: 79.2%) Ή-NMR (500 MHz, CDC13): 8.02-7.99 (m, 1H), 7.45-7.40 (m, lH), 7.00-6.95 (m, 2H), 5.16 (s, lH), 3.74 (s, 3H), 3.76 (s, 3H)
PAT
https://patents.google.com/patent/WO2021256861A1/en
PAT
https://patents.google.com/patent/CN112094219A/en

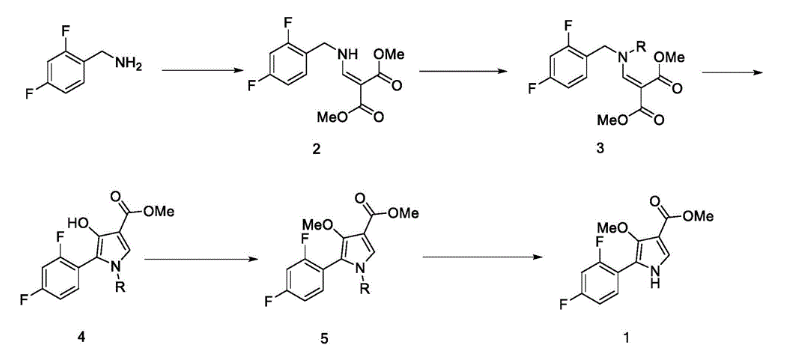
Synthesis of Compound 1
In a 500ml reaction flask were charged 10 g of compound 5B, 100 ml of acetonitrile, 50 ml of water, 56 g of ceric ammonium nitrate, and reacted at room temperature for 12 hours. 100 ml of water and 100 ml of ethyl acetate are added. The mixture was allowed to stand for separation, and the aqueous layer was extracted twice with ethyl acetate. The combined organic layers were washed with water and saturated brine in this order, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give a crude product of Compound 1. The crude product was crystallized from ethyl acetate and n-heptane to give 6.1 g of compound 1 in 85.6% yield as a pale yellow solid.
Syn
https://pubs.acs.org/doi/10.1021/acs.oprd.5c00255



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Ramani A, Merchant A, Cash BD (August 2023). “Review of the clinical development of fexuprazan for gastroesophageal reflux-related disease”. European Journal of Clinical Pharmacology. 79 (8): 1023–1029. doi:10.1007/s00228-023-03521-4. PMID 37344679. S2CID 259222741.
- Kim GH, Choi MG, Kim JI, Lee ST, Chun HJ, Lee KL, et al. (November 2023). “Efficacy and Safety of Fexuprazan in Patients with Acute or Chronic Gastritis”. Gut and Liver. 17 (6): 884–893. doi:10.5009/gnl220457. PMC 10651377. PMID 36789577.
- Jeong YS, Kim MS, Lee N, Lee A, Chae YJ, Chung SJ, et al. (May 2021). “Development of Physiologically Based Pharmacokinetic Model for Orally Administered Fexuprazan in Humans”. Pharmaceutics. 13 (6): 813. doi:10.3390/pharmaceutics13060813. PMC 8229463. PMID 34072547.
- Kim MS, Lee N, Lee A, Chae YJ, Chung SJ, Lee KR (June 2022). “Model-Based Prediction of Acid Suppression and Proposal of a New Dosing Regimen of Fexuprazan in Humans”. Pharmaceuticals. 15 (6): 709. doi:10.3390/ph15060709. PMC 9230547. PMID 35745628.
- “펙수클루정40밀리그램(펙수프라잔염산염)” [Fexuclue tablets 40 mg (fexuprazan hydrochloride)]. nedrug.mfds.go.kr (in Korean).
- “Daewoong Pharma’s GER drug gets product OK from Mexico”. Korea Economic Daily. 19 October 2023.
- Park IH. “Daewoong launches GERD treatment Fexuclu in Philippines”. KED Global. Retrieved 4 April 2025.
- Lee JH (14 March 2023). “Daewoong wins approval for GERD treatment Fexuclu in Chile”. KED Global. Retrieved 4 April 2025.
- Kim JE. “Daewoong receives approval for its GERD drug Fexuclue in Ecuador”. KED Global. Retrieved 4 April 2025.
| Clinical data | |
|---|---|
| Trade names | Fexuclue |
| Other names | Abeprazan; DWP14012; DWP-14012 |
| ATC code | A02BC10 (WHO) |
| Legal status | |
| Legal status | Rx in South Korea, Mexico |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1902954-60-2 |
| PubChem CID | 122662112 |
| DrugBank | DB16078 |
| ChemSpider | 68006985 |
| UNII | BE52S2C1QT |
| KEGG | D13012 |
| ChEMBL | ChEMBL4594445 |
| Chemical and physical data | |
| Formula | C19H17F3N2O3S |
| Molar mass | 410.41 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
////////Fexuprazan, Abeprazan, DWP14012, DWP-14012














