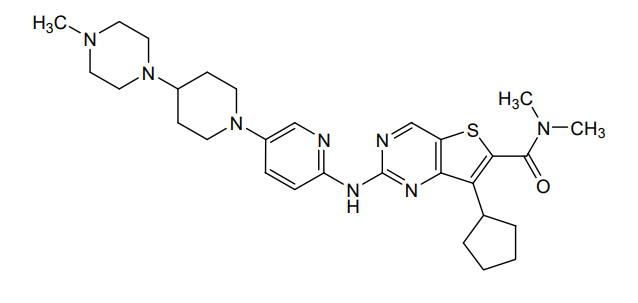
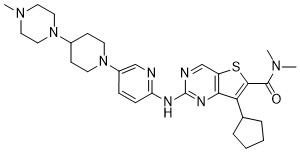
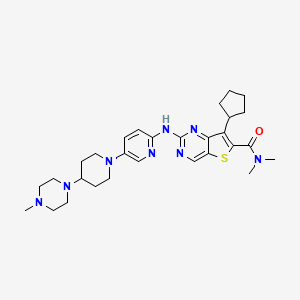
Fovinaciclib
CAS 2146171-49-3
MF C29H40N8OS
Exact Mass: 548.3046
Molecular Weight: 548.75
7-cyclopentyl-N,N-dimethyl-2-({5-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]pyridin-2-yl}amino) thieno[3,2-d]pyrimidine-6-carboxamide
7-cyclopentyl-N,N-dimethyl-2-((5-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridin-2-yl)amino)thieno[3,2-d]pyrimidine-6-carboxamide
7-cyclopentyl-N, N-dimethyl-2- ( (5- (4- (4-methylpiperazin-1-yl) piperidin-1-yl) pyridin-2-yl) amino) thieno [3, 2-d] pyrimidine-6-carboxamide
7-Cyclopentyl-N,N-dimethyl-2-((5-(4-(1-methylpiperidin-4-yl)piperazin-1-yl)pyridin-2-yl)amino Thieno[3,2-d]pyrimidine-6-carboxamide
cyclin dependent kinase inhibitor, antineoplastic, Fovinaciclibum, LPW3H579X8, inzhou Aohong Pharmaceutical Co
- OriginatorChongqing Fochon Pharmaceutical
- DeveloperAhon Pharmaceutical; Chongqing Fochon Pharmaceutical; Shanghai Fosun Pharmaceutical
- Class2 ring heterocyclic compounds; Amides; Amines; Antineoplastics; Cyclopentanes; Piperazines; Piperidines; Pyridines; Pyrimidines; Small molecules; Thiophenes
- Mechanism of ActionCyclin-dependent kinase 4 inhibitors; Cyclin-dependent kinase 6 inhibitors
- MarketedHER2 negative breast cancer
- No development reportedSolid tumours
- 04 Sep 2025Chemical structure information added.
- 02 Sep 2025Launched for HER2-negative-breast-cancer (Late-stage disease, Second-line therapy or greater) in China (PO) (Shanghai Henlius Biotech pipeline, September 2025)
- 26 Aug 2025Registered for HER2-negative-breast-cancer (Late-stage disease, Second-line therapy or greater) in China (PO) prior to August 2025
Fovinaciclib is an orally bioavailable inhibitor of cyclin-dependent kinase (CDK) types 4 (CDK4) and 6 (CDK6), with potential antineoplastic activity. Upon administration, fovinaciclib selectively inhibits CDK4 and CDK6, which inhibits the phosphorylation of retinoblastoma protein (Rb) early in the G1 phase, prevents CDK-mediated G1/S transition and leads to cell cycle arrest. This suppresses DNA replication and decreases tumor cell proliferation. CDK4 and 6 are serine/threonine kinases that are upregulated in many tumor cell types and play key roles in the regulation of both cell cycle progression from the G1-phase into the S-phase and cell proliferation.
On May 29, 2025, China’s National Medical Products Administration (NMPA) approved the Class 1 innovative drug Fovinaciclib (CDK4&6 inhibitor), developed by Jinzhou Aohong Pharmaceutical Co., Ltd. This medication, in combination with fulvestrant, is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative recurrent or metastatic breast cancer, who have experienced disease progression following prior endocrine therapy.
Notably, Fovinaciclib represents an excellent example of scaffold hopping—its design replaces the pyrrolo-pyrimidine core of Ribociclib (first approved on March 13, 2017) with a thieno-pyrimidine ring.
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN236278427&_cid=P21-MGRD95-18783-1
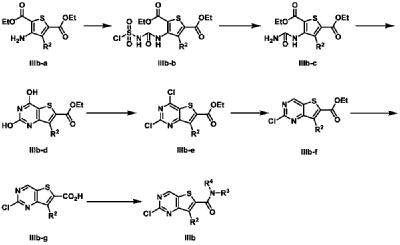
| Example 3 |
| 7-Cyclopentyl-N,N-dimethyl-2-((5-(4-(1-methylpiperidin-4-yl)piperazin-1-yl)pyridin-2-yl)amino Thieno[3,2-d]pyrimidine-6-carboxamide (3) |
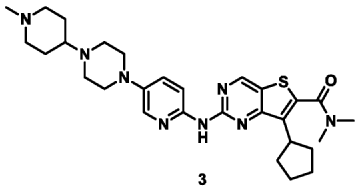
According to the synthesis method of Example 2, CH
3 CHO replaced by CH
2 O, to prepare the title compound 7-cyclopentyl-N,N-dimethyl-2-((5-(4-(1-methylpiperidin-4-yl)piperazin-1-yl)pyridin-2-yl)amino)thieno[3,2-d]pyrimidine-6-carboxamide (3). MS-ESI (m/z): 549 [M+1] + .
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017193872&_cid=P21-MGRDEF-24321-1
[0266]
7-cyclopentyl-N, N-dimethyl-2- ( (5- (4- (4-methylpiperazin-1-yl) piperidin-1-yl) pyridi n-2-yl) amino) thieno [3, 2-d] pyrimidine-6-carboxamide (5)
[0267]
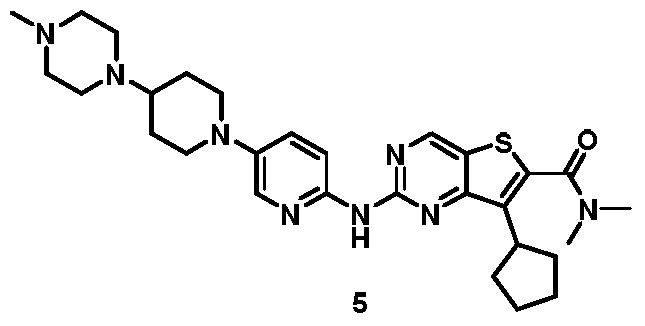
To a solution of 7-cyclopentyl-N, N-dimethyl-2- ( (5- (4- (piperazin-1-yl) piperidin-1-yl) pyridin-2-yl) amino) thieno [3, 2-d] pyrimidine-6-carboxamide (4) (1.5 g, 2.8 mmol) in DCM (45 mL) was added NaBH (OAc) 3(3.56 mg, 16.8 mmol) followed by CH 2O (40%in water, 252 mg, 3.4 mmol) . The mixture was stirred at r.t. for 30 min. The mixture was diluted with saturated aqueous NaHCO 3(100 mL) and extracted with DCM (2 × 30 mL) . The extracts were dried over Na 2SO 4. Solvents were evaporated under reduced pressure. The residue was purified by column chromatography on silica gel eluting with 96: 3: 1 DCM/methanol/ammonia to give 7-cyclopentyl-N, N-dimethyl-2- ( (5- (4- (4-methylpiperazin-1-yl) piperidin-1-yl) pyridin-2-yl) amino) thieno [3, 2-d] pyrimidine-6-carboxamide (5) . MS-ESI (m/z) : 549 [M + 1] +.
PAT
- Certain protein kinase inhibitorsPublication Number: JP-2019516790-APriority Date: 2016-05-07
- Certain protein kinase inhibitorsPublication Number: US-2019209566-A1Priority Date: 2016-05-07
- Certain protein kinase inhibitorsPublication Number: WO-2017193872-A1Priority Date: 2016-05-07
- Certain protein kinase inhibitorsPublication Number: US-10835535-B2Priority Date: 2016-05-07Grant Date: 2020-11-17
- A class of protein kinase inhibitorsPublication Number: CN-109153686-BPriority Date: 2016-05-07Grant Date: 2021-04-30
- specific protein kinase inhibitorsPublication Number: KR-102374033-B1Priority Date: 2016-05-07Grant Date: 2022-03-14
- Certain protein kinase inhibitorsPublication Number: EP-3452484-B1Priority Date: 2016-05-07Grant Date: 2023-07-05
- Certain protein kinase inhibitorsPublication Number: ES-2954148-T3Priority Date: 2016-05-07Grant Date: 2023-11-20



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
//////////Fovinaciclib, CHINA 2025, APPROVALS 2025, cyclin dependent kinase inhibitor, antineoplastic, Fovinaciclibum, LPW3H579X8, inzhou Aohong Pharmaceutical Co














