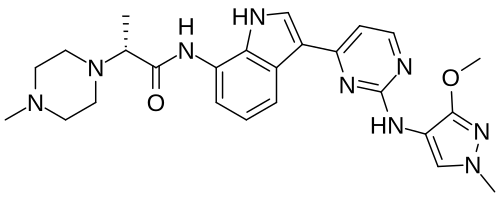
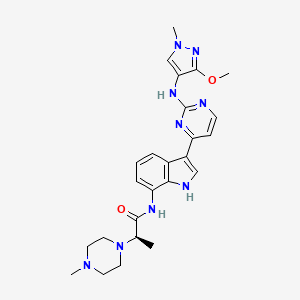
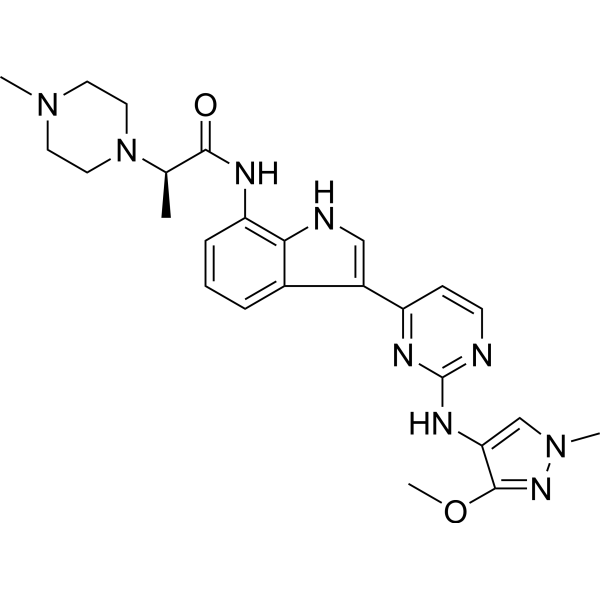
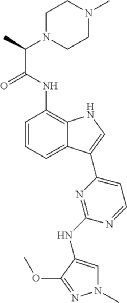
Golidocitinib
CAS 2091134-68-6
- AZD-4205
- AZD4205
- UNII-3BY9Z3M34G
- 3BY9Z3M34G
WeightAverage: 489.584
Monoisotopic: 489.260071274
Chemical FormulaC25H31N9O2
(2R)-N-[3-[2-[(3-methoxy-1-methylpyrazol-4-yl)amino]pyrimidin-4-yl]-1H-indol-7-yl]-2-(4-methylpiperazin-1-yl)propanamide
- (2R)-N-(3-(2-((3-methoxy-1-methylpyrazol-4-yl)amino)pyrimidin-4-yl)-1H-indol-7-yl)-2-(4-methylpiperazin-1-yl)propanamide
- (ALPHAR)-N-(3-(2-((3-METHOXY-1-METHYL-1H-PYRAZOL-4-YL)AMINO)-4-PYRIMIDINYL)-1H-INDOL-7-YL)-ALPHA,4-DIMETHYL-1-PIPERAZINEACETAMIDE
- (2R)-N-[3-[2-[(3-Methoxy-1-methyl-pyrazol-4-yl)amino]pyrimidin-4-yl]-1H-indol-7-yl]-2-(4-methylpiperazin-1-yl)propenamide
- (R)-N-(3-(2-(3-Methoxy-1-methyl-1H-pyrazol-4-ylamino)pyrimidin-4-yl)-1H-indol-7-yl)-2-(4-methylpiperazin-1-yl)propanamide
Approvals 2024, china 2024, DZD 4205, DIZAL, Gao Ruizhe,
Golidocitinib is a pharmaceutical drug for the treatment of cancer. In June 2024, it was given conditional approval in China for the treatment of relapsed or refractory peripheral T-cell lymphoma.[1]
Golidocitinib is classified as a Janus kinase inhibitor.[2][3]
Golidocitinib is an orally available inhibitor of Janus-associated kinase 1 (JAK1), with potential antineoplastic activity. Upon oral administration, golidocitinib inhibits JAK-dependent signaling and may lead to an inhibition of cellular proliferation in JAK1-overexpressing tumor cells. The JAK-STAT (signal transducer and activator of transcription) signaling pathway is a major mediator of cytokine activity and is often dysregulated in a variety of tumor cell types. Additionally, JAK1 may be a primary driver of STAT3 phosphorylation and signaling, which plays a role in neoplastic transformation, resistance to apoptosis, tumor angiogenesis, metastasis, immune evasion, and treatment resistance.
GOLIDOCITINIB is a small molecule drug with a maximum clinical trial phase of II (across all indications) and has 4 investigational indications.
PAT
US9714236, https://patentscope.wipo.int/search/en/detail.jsf?docId=US193702885&_cid=P11-MEHX78-54823-1
Example 32: (2R)—N-(3-{2-[(3-Methoxy-1-methyl-1H-pyrazol-4-yl)amino]pyrimidin-4-yl}-1H-indol-7-yl)-2-(4-methylpiperazin-1-yl)propanamide
| The procedure described above for Example 32 was repeated using the indicated Intermediates to give Examples 33-42 described in Table 12: |
[TABLE-US-00012]
| TABLE 12 Starting m/z ExampleIntermediatesNMR δ (400 MHz)[M + H]+Yield % 3325 and 38DMSO-d6 with D2O 1.28 (3H, d), 2.2750413 (3H, s), 2.73 (3H, s), 2.85-3.34 (8H, m), 3.44 (1H, q), 3.63 (3H, s), 374 (3H, s), 7.04 (1H, t), 7.19 (1H, d), 7.55 (1H, s), 7.91 (1H, s), 8.08 (2H, s), 8.26 (1H, s) -two exchangeable protons not observed3425 and 37DMSO-d6 1.26 (3H, d), 2.16 (3H, s),50472 2.33 (3H, s), 2.38 (4H, s), 2.57-2.62 (4H, m), 3.33 (1H, q), 3.67 (3H, s), 3.79 (3H, s), 7.00 (1H, t), 7.41 (1H, d), 7.66 (1H, s), 7.96 (2H, t), 8.14 (1H, s), 8.22 (1H, s), 9.65 (1H, s), 11.28 (1H, s)3530 and 37Methanol-d4 1.34 (3H, t), 1.40 (3H, d),51816 2.32 (3H, s), 2.37 (3H, s), 2.50-2.80 (8H, m), 3.38 (1H, q), 3.69 (3H, s), 4.34 (2H, q), 7.05-7.20 (2H, m), 7.69 (1H, s), 7.85 (1H, s), 8.23 (1H, s), 8.17 (1H, d)-three exchangeable protons not observed3626 and 37DMSO-d6 1.26 (3H, d), 2.27 (3H, s),52448 2.24-2.52 (4H, m), 2.53-2.70 (4H, m), 3.30-3.36 (1H, m), 3.69 (3H, s), 3.78 (3H, s), 7.02 (1H, s), 7.40 (1H, d), 7.65 (1H, s), 8.32 (1H, s), 8.48 (1H, s), 9.69 (1H, s), 11.42 (1H, s)3727 and 37DMSO-d6 1.26 (3H, d), 2.17 (3H, s),56849 2.23-2.45 (4H, m), 2.46-2.71 (4H, m), 3.30-3.32 (1H, m), 3.68 (3H, s), 3.78 (3H, s), 7.01 (1H, s), 7.37 (1H, d), 7.64 (1H, s), 8.42 (1H, s), 8.45-8.56 (2H, m), 9.70 (1H, s), 11.36 (1H, s)3825 and 39Chloroform-d 1.19 (3H, d), 1.35 (3H, d),51819 2.10 (1H, m), 2.26 (1H, m), 2.38 (6H, m), 2.69 (2H, t), 2.89 (3H, m), 3.72 (3H, s), 3.91 (1H, q), 4.00 (3H, s), 6.57 (1H, s), 6.80 (1H, d), 7.15 (1H, t), 7.68 (1H, d), 7.84 (1H, s), 8.06-8.36 (2H, m), 9.88 (1H, s), 11.15 (1H, s)3929 and 37Methanol-d4 1.34 (3H, t), 1.43 (3H, d),52225 2.35 (3H, s), 2.50-2.85 (8H, m), 3.41 (1H, q), 3.79 (3H, s), 4.24 (2H, q), 7.10- 7.22 (2H, m), 7.68 (1H, s), 8.13 (1H, d), 8.16 (1H, d), 8.43 (1H, s)-three exchangeable protons not observed4031 and 37Methanol-d4 1.33 (3H, t), 1.42 (3H, d),53822 2.35 (3H, s), 2.63-2.71 (4H, m), 2.77- 2.81 (4H, m), 3.42 (1H, q), 3.76 (3H, s), 4.26 (2H, q), 7.10-7.20 (2H, m), 7.70 (1H, s), 8.28 (2H, m), 8.48 (1H, m)-three exchangeable protons not observed4128 and 37Chloroform-d 1.41 (3H, d), 2.29 (3H, s),48836 2.36 (3H, s), 2.42 (3H, s), 2.67-2.80 (8H, m), 3.38 (1H, q), 3.80 (3H, s), 6.42 (1H, s), 6.82 (1H, d), 7.12 (1H, t), 7.69 (1H, d), 7.88 (1H, s), 8.21 (2H, m), 9.74 (1H, s), 11.18 (1H, s)4228 and 38DMSO-d6 1.27 (3H, d), 2.12 (3H, s),4884 2.17 (3H, s), 2.35 (3H, s), 2.40 (4H, s), 2.57-2.63 (4H, m), 3.72 (3H, s), 7.03 (1H, t), 7.43 (1H, d), 7.81 (1H, s), 7.97 (1H, d), 8.19 (2H, m), 8.37 (1H, s), 9.68 (1H, s), 11.33 (1H, s) |
SYN
CN108368091
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN225024309&_cid=P11-MEHXD5-59000-1
| Example 32: (2R)-N-(3-{2-[(3-methoxy-1-methyl-1H-pyrazol-4-yl)amino]pyrimidin-4-yl}-1H-indol-7-yl)-2-(4-methylpiperazin-1-yl)propanamide |
| |
| 3-{2-[(3-methoxy-1-methyl-1H-pyrazol-4-yl)amino]pyrimidin-4-yl}-1H-indol-7-amine (180 mg, 0.54 mmol, Intermediate 23), (R)-2-(4-methylpiperazin-1-yl)propanoic acid dihydrochloride (158 mg, 0.64 mmol, Intermediate 37) and HATU (408 mg, 1.1 mmol) were stirred together in THF (5 mL) to give an orange solution. Diisopropylethylamine (0.38 mL, 2.2 mmol) was added at 25°C. The resulting suspension was stirred at 25°C for 3 hours. The reaction mixture was diluted with ethyl acetate (100 mL) and washed with saturated NaCl. 2 CO 3 The mixture was stirred for 2 hours at 4 ℃ for 10 minutes.Then the mixture was stirred for 2 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 10 minutes.Then the mixture was stirred for 2 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 10 minutes.Then the mixture was stirred for 2 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 4 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 4 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 3 hours.Then the mixture was stirred for 4 hours.Then the mixture was stirred for 3 hours . δ (DMSO, 400 MHz) 1.26 (3H, d), 2.16 (3H, s), 2.25-2.45 (4H, m), 2.51-2.70 (4H, m), 3.71 (3H, s), 3.80 (3H, s), 7.05 (1H, t), 7.13 (1H, d), 7.38 (1H, d), 7.70 (1H, s), 8.16-8.31 (4H, m), 9.62 (1H, s), 11.35 (1H, s) – the α-proton of the amide is obscured by the residual water peak; m/z (ES+), [M+H]+=490. |
| The above procedure for Example 32 was repeated using the indicated intermediates to obtain Examples 33-42 described in Table 12: |
SYN
European Journal of Medicinal Chemistry 291 (2025) 117643
Golidocitinib, also known as DZD4205, is an oral, selective Janus kinase 1 (JAK1) inhibitor developed by Dizal Pharmaceutical. It is designed to target aberrant JAK/STAT signaling pathways implicated in
various malignancies, particularly peripheral T-cell lymphoma (PTCL) [31]. In 2024, Golidocitinib was granted conditional approval by the NMPA under the brand name Gao Ruizhe, for the treatment of adult patients with relapsed or refractory PTCL who have received at least one line of systemic therapy. This agent exerts its therapeutic effects through selective inhibition of JAK1, thereby disrupting the JAK/STAT signaling pathway [32]. This inhibition leads to reduced proliferation and increased apoptosis of malignant T-cells in PTCL [33]. The clinical efficacy of Golidocitinib was demonstrated in the Phase II JACKPOT8 Part B study (NCT04105010), a multinational, single-arm trial evaluating its use in patients with r/r PTCL [34]. The investigation demonstrated an ORR of 44.3 % in patients with PTCL, with sustained efficacy noted across diverse PTCL subtypes. In terms of safety profile, Golidocitinib exhibited favorable tolerability. Hematologic adverse events such as anemia, neutropenia, and thrombocytopenia were the predominant treatment-related toxicities, yet they were effectively controlled through dose modifications and supportive interventions.
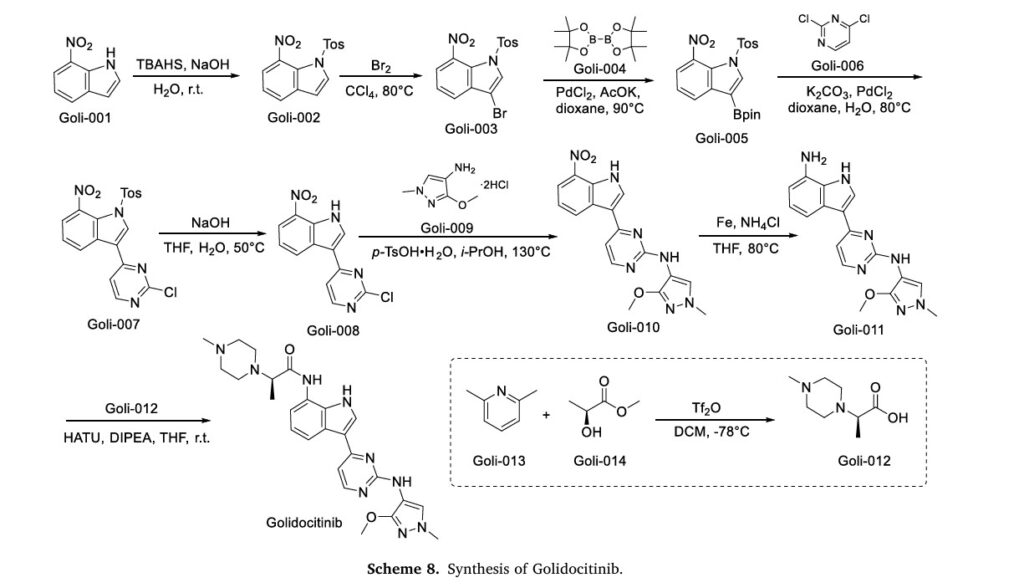
The synthetic route of Golidocitinib, shown in Scheme 8, initiates with amino protection of Goli-001 to afford Goli-002 [35]. Bromination of Goli-002 with Br2 yields Goli-003, which undergoes Miyaura bor
ylation with Goli-004 to form Goli-005. Suzuki-Miyaura coupling of Goli-005 with Goli-006 generates Goli-007. Deprotection of Goli-007 produces Goli-008, which undergoes p-TsOH-mediated nucleophilic
substitution with Goli-009 to yield Goli-010. Reduction of Goli-010 affords Goli-011, followed by amidation with Goli-012 to deliver Golidocitinib. Concurrently, Goli-012 is prepared via Tf2 0- Mediated
nucleophilic substitution between Goli-013 and Goli-014.
[31] S.J. Keam, Golidocitinib: first approval, Drugs 84 (2024) 1319–1324.
[32] K. Chen, X. Guan, Z. Yang, Y. Zhou, Z. Liu, X. Deng, D. Liu, P. Hu, R. Chen,
Pharmacokinetic characteristics of golidocitinib, a highly selective JAK1 inhibitor,
in healthy adult participants, Front. Immunol. 14 (2023) 1127935.
[33] M.B. Nierengarten, Golidocitinib favorable for relapsed/refractory T-cell
lymphoma, Cancer 130 (2024) 1191–1192.
[34] Y. Song, L. Malpica, Q. Cai, W. Zhao, K. Zhou, J. Wu, H. Zhang, N. Mehta-Shah,
K. Ding, Y. Liu, Z. Li, L. Zhang, M. Zheng, J. Jin, H. Yang, Y. Shuang, D.H. Yoon,
S. Gao, W. Li, Z. Zhai, L. Zou, Y. Xi, Y. Koh, F. Li, M. Prince, H. Zhou, L. Lin, H. Liu,
P. Allen, F. Roncolato, Z. Yang, W.S. Kim, J. Zhu, Golidocitinib, a selective JAK1
tyrosine-kinase inhibitor, in patients with refractory or relapsed peripheral T-cell
lymphoma (JACKPOT8 part B): a single-arm, multinational, phase 2 study, Lancet
Oncol. 25 (2024) 117–125.
[35] A.B.M. Aastrand, N.P. Grimster, S. Kawatkar, J.G. Kettle, M.K. Nilsson, L.L. Ruston,
Q. Su, M.M. Vasbinder, J.J. Winter-Holt, D. Wu, W. Yang, T. Grecu, J. McCabe, R.
D. Woessner, C.E. Chuaqui, Preparation of Substituted 2-(piperazin-1-yl)-N-[3-[2-
[(1H-pyrazol-4-yl)amino]pyrimidin-4-yl]-1H-indol-7-yl] Propanamide as Selective
JAK1 Inhibitors for Treating Cancers and Immune Disorders, 2017
CN108368091A.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Keam SJ (October 2024). “Golidocitinib: First Approval”. Drugs. 84 (10): 1319–1324. doi:10.1007/s40265-024-02089-2. PMID 39298087.
- Song Y, Malpica L, Cai Q, Zhao W, Zhou K, Wu J, et al. (January 2024). “Golidocitinib, a selective JAK1 tyrosine-kinase inhibitor, in patients with refractory or relapsed peripheral T-cell lymphoma (JACKPOT8 Part B): a single-arm, multinational, phase 2 study”. The Lancet. Oncology. 25 (1): 117–125. doi:10.1016/S1470-2045(23)00589-2. PMID 38092009.
- Jin J, Zhang L, Zou L, Li Z, Wu H, Zhou K, et al. (2024). “Maintenance Therapy of Golidocitinib, a JAK1 Selective Inhibitor, in Patients with Peripheral T Cell Lymphomas after First-Line Systemic Therapy: Updates of the Phase 2 Study (JACKPOT26)”. Blood. 144: 6368. doi:10.1182/blood-2024-211891.
| Clinical data | |
|---|---|
| Trade names | 高瑞哲 (Gao Ruizhe) |
| Other names | AZD-4205, AZD4205, JAK1-IN-3 |
| Legal status | |
| Legal status | Rx in China |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2091134-68-6 |
| PubChem CID | 126715380 |
| DrugBank | DB18057 |
| ChemSpider | 71117616 |
| UNII | 3BY9Z3M34G |
| KEGG | D12502 |
| ChEMBL | ChEMBL4577523 |
| Chemical and physical data | |
| Formula | C25H31N9O2 |
| Molar mass | 489.584 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
- New drugs approved by the NMPA in 2024: Synthesis and clinical applicationsPublication Name: European Journal of Medicinal ChemistryPublication Date: 2025-07-05PMID: 40262297DOI: 10.1016/j.ejmech.2025.117643
- Golidocitinib: First ApprovalPublication Name: DrugsPublication Date: 2024-09-19PMID: 39298087DOI: 10.1007/s40265-024-02089-2
- Recent Developments in the Use of Kinase Inhibitors for Management of Viral InfectionsPublication Name: Journal of Medicinal ChemistryPublication Date: 2021-02-04PMID: 33539089DOI: 10.1021/acs.jmedchem.0c01467
- Discovery of (2R)-N-[3-[2-[(3-Methoxy-1-methyl-pyrazol-4-yl)amino]pyrimidin-4-yl]-1H-indol-7-yl]-2-(4-methylpiperazin-1-yl)propenamide (AZD4205) as a Potent and Selective Janus Kinase 1 InhibitorPublication Name: Journal of Medicinal ChemistryPublication Date: 2020-04-16PMID: 32297743DOI: 10.1021/acs.jmedchem.9b01392
- Sexuality in a healthcare settingPublication Name: Modern healthcare. [Short-term care ed.]Publication Date: 1976-05PMID: 5656
//////////Golidocitinib, approvals 2024, china 2024, DZD 4205, DIZAL, Gao Ruizhe, AZD-4205, AZD4205, UNII-3BY9Z3M34G, 3BY9Z3M34G














