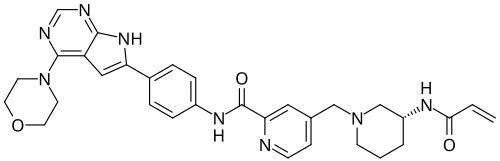
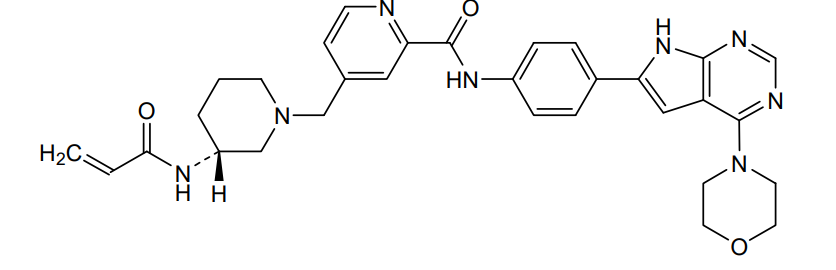
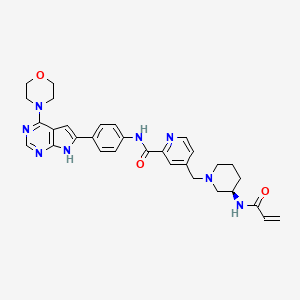
Icovamenib
CAS 2448172-22-1
MF C31H34N8O3 MW 566.7 g/mol
N-{4-[4-(morpholin-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-6-yl]phenyl}-4-{[(3R)-3-(prop-2-enamido) piperidin-1-yl]methyl}pyridine-2-carboxamide
N-[4-(4-morpholin-4-yl-7H-pyrrolo[2,3-d]pyrimidin-6-yl)phenyl]-4-[[(3R)-3-(prop-2-enoylamino)piperidin-1-yl]methyl]pyridine-2-carboxamide
menin-MLL (mixed-lineage leukemia) protein interaction inhibitor,
antineoplastic, BMF-219, BMF 219, 2Z737MY35A, Menin-MLL inhibitor 21
Icovamenib is an investigational irreversible covalent inhibitor of menin. It is developed by Biomea Fusion for diabetes, lymphoma, leukemia, and multiple myeloma.[1][2][3]
Icovamenib is an orally bioavailable, irriversible inhibitor of menin, an essential co-factor of oncogenic menin-mixed lineage leukemia (MLL; myeloid/lymphoid leukemia; KMT2A) fusion proteins, with potential antineoplastic activity. Upon oral administration, icovamenib specifically targets and binds to menin, thereby preventing the interaction between the two proteins menin and MLL and the formation of the menin-MLL complex. This reduces the expression of downstream target genes, such as MYC and Bcl2, and results in an inhibition of the proliferation of MLL-rearranged tumor cells. Menin, an essential transcriptional regulator, plays a key role in oncogenic signaling in cancers driven by oncogenic MLL-fusions.
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US299042443&_cid=P20-MH9YDY-31032-1
Example 9
Synthesis of Compound 10
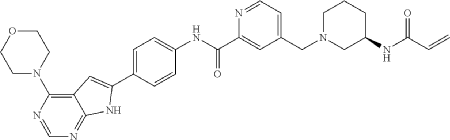
Compound 10
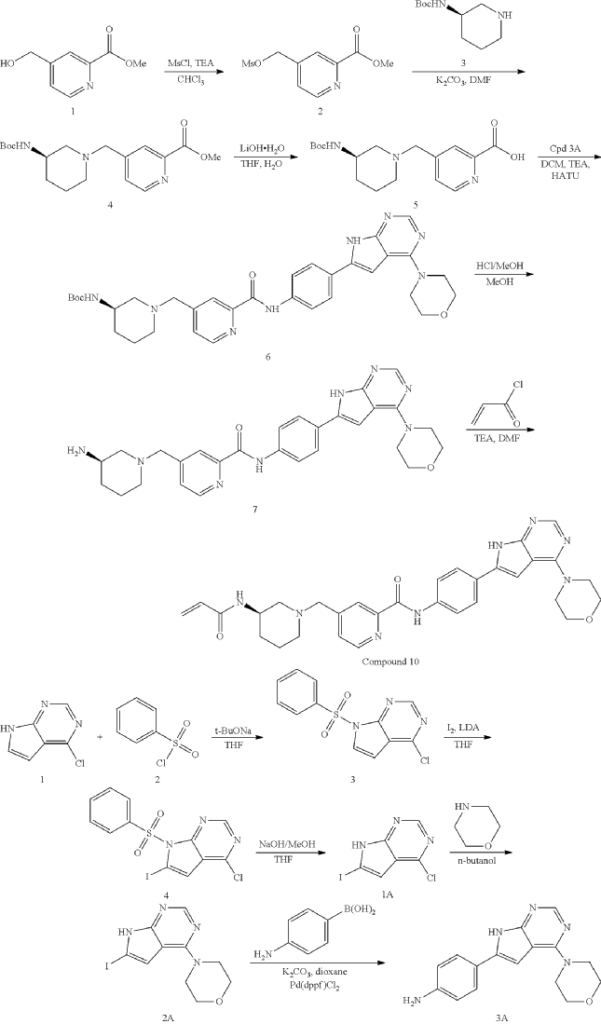
General Procedure for Preparation of Intermediate 2
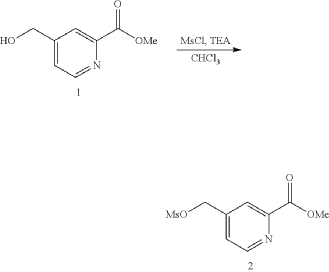
| 1H NMR: CDCl 3 400 MHz 8.80 (d, J=4.85 Hz, 1H), 8.15 (d, J=0.66 Hz, 1H), 7.53 (dt, J=4.91, 0.85 Hz, 1H), 5.27-5.34 (m, 2H), 4.00-4.08 (m, 3H), 3.11 (s, 3H) |
General Procedure for Preparation of Intermediate 5—
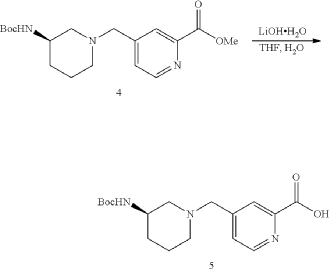
| To a solution of Intermediate 4 (1.50 g, 4.29 mmol, 1 eq) in THF (7.00 mL) was added LiOH.H 2O (540.3 mg, 12.8 mmol, 3 eq) in H 2O (7.00 mL). The mixture was stirred at 25° C. for 3 h. TLC (Dichloromethane:Methanol=10:1, R f=0) showed the reaction was complete. The mixture was poured into H 2O (20.0 mL) and extracted with DCM (10.0 mL×3). Then the organic phases dried over Na 2SO 4, filtered and concentrated under vacuum. The crude without purification. Give the Intermediate 5 (1.20 g, crude) as a yellow solid. |
General Procedure for Preparation of Intermediate 6—
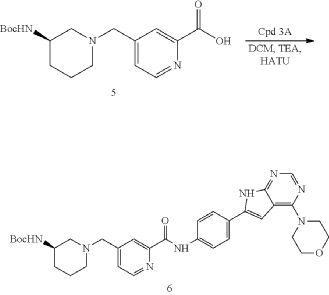
| To a solution of Intermediate 5 (0.80 g, 2.39 mmol, 1 eq), Intermediate 3A (704.4 mg, 2.39 mmol, 1 eq), TEA (1.69 g, 16.7 mmol, 2.32 mL, 7 eq) in DCM (10.0 mL) was added HATU (1.36 g, 3.58 mmol, 1.5 eq). The mixture was stirred at 20° C. for 12 h. LCMS showed the reaction was complete. The mixture was poured into H 2O (40.0 mL) and extracted with DCM (20.0 mL×3). Then the organic phases were washed with brine (50.0 mL) dried over Na 2SO 4, filtered and concentrated under vacuum. The crude for next step without purification. Give the Intermediate 6 (0.60 g, crude) as a yellow solid. |
General Procedure for Preparation of Intermediate 7—
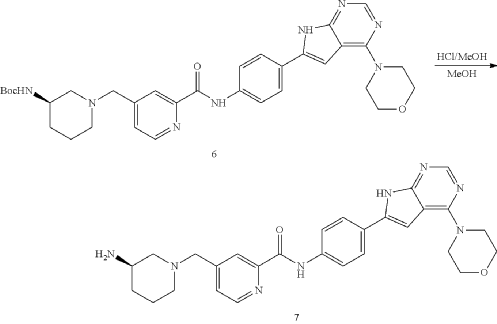
| To a solution of Intermediate 6 (0.50 g, 816.0 umol, 1 eq) in MeOH (5.00 mL) was added HCl/MeOH (4 M, 5.00 mL, 24.51 eq). The mixture was stirred at 20° C. for 12 h. LCMS showed the reaction was complete. The mixture was concentrated under vacuum. The crude for next step without purification. Give the Intermediate 7 (0.50 g, crude, HCl) as a yellow solid. |
General Procedure for Preparation of Compound 10—
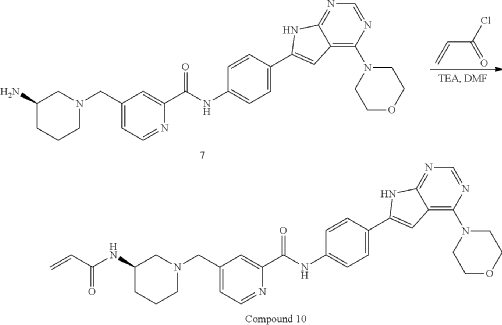
| To a solution of Intermediate 3 (0.50 g, 910.6 umol, 1 eq, HCl) in DMF (10.0 mL) was added TEA (645.0 mg, 6.37 mmol, 887.2 uL, 7 eq) and prop-2-enoyl chloride (82.4 mg, 910.6 umol, 74.2 uL, 1 eq). Then the mixture was stirred at 20° C. for 12 h. LCMS showed the reaction was complete. The mixture was poured into H 2O (50.0 mL), then was filtered and filter cake was concentrated in vacuum. The crude product was purified by reversed-phase HPLC (column: Phenomenex Luna C18 200*40 mm*10 um; mobile phase: [water(0.05% HCl)-ACN]; B %: 10%-30%, 10 min) and (column: Xtimate C18 150*25 mm*5 um; mobile phase: [water(10 mM NH 4HCO 3)-ACN]; B %: 30%-60%, 10 min). Give the Intermediate Compound 10 (20.0 mg, 35.0 umol, 3.85% yield, 99.3% purity) as a yellow solid. |
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2024172911&_cid=P20-MH9YNT-37455-1
PAT
- Substituted pyridines as irreversible inhibitors of menin-MLL interactionPublication Number: US-11702421-B2Priority Date: 2018-12-31Grant Date: 2023-07-18
- Irreversible inhibitors of menin-mll interactionPublication Number: US-2023227458-A1Priority Date: 2018-12-31
- N-[4-[4-(4-MORPHOLINYL)-7H-PYRROLO[2,3-d]PYRIMIDIN-6-YL]PHENYL]-4-[[3(R)-[(1-OXO-2-PROPEN-1-YL)AMINO]-1-PIPERIDINYL]METHYL]-2-PYRIDINE CARBOXAMIDE AND USES THEREOFPublication Number: US-2024376112-A1Priority Date: 2018-12-31
- Covalent inhibitors of menin-mll interaction for diabetes mellitusPublication Number: WO-2023018825-A1Priority Date: 2021-08-11
- Covalent inhibitors of Mennin-MLL interaction for diabetesPublication Number: CN-118076357-APriority Date: 2021-08-11
- Irreversible inhibitors of menin-mll interactionPublication Number: US-2020223853-A1Priority Date: 2018-12-31
- Substituted pyridines as irreversible inhibitors of menin-MLL interactionPublication Number: US-11084825-B2Priority Date: 2018-12-31Grant Date: 2021-08-10
- Irreversible inhibitors of menin-mll interactionPublication Number: US-2022169627-A1Priority Date: 2018-12-31
- Crystalline forms of an irreversible inhibitor of menin-mll interactionPublication Number: US-2023086137-A1Priority Date: 2021-08-20
- Crystalline form of n-[4-[4-(4-morpholinyl)-7h-pyrrolo[2,3-d]pyrimidin-6-yl]phenyl]-4-[[3(r)-[(1-oxo -2-propen-1-yl)amino]-1-piperidinyl]methyl]-2-pyridinecarboxamide, an irreversible menin-mll inhibitor for the treatment of cancerPublication Number: EP-4387972-A1Priority Date: 2021-08-20
- Crystalline forms of N-[4-[4-(4-morpholinyl)-7H-pyrrolo[2,3-d]pyrimidin-6-yl]phenyl]-4-[[3(r)-[(1-oxo-2-propen-1-yl)amino]-1-piperidinyl]methyl]-2-pyridinecarboxamide as an irreversible inhibitor of menin-MLL interactionPublication Number: US-12018032-B2Priority Date: 2021-08-20Grant Date: 2024-06-25
- Crystalline form of n-[4-[4-(4-morpholinyl)-7h-pyrrolo[2,3-d]pyrimidin-6-yl]phenyl]-4-[[3(r)-[(1-oxo -2-propen-1-yl)amino]-1-piperidinyl]methyl]-2-pyridinecarboxamide, an irreversible menin-mll inhibitor for the treatment of cancerPublication Number: WO-2023022912-A1Priority Date: 2021-08-20
- Crystalline forms of an irreversible inhibitor of menin-mll interactionPublication Number: US-2024343731-A1Priority Date: 2021-08-20
- Synthetic methods for preparing a pyridinecarboxamide compoundPublication Number: WO-2024011450-A1Priority Date: 2022-07-13
- Menin-mll inhibitors and compositions for proliferation of beta cellsPublication Number: WO-2024006391-A1Priority Date: 2022-06-28
- Flt3 combination therapy for cancer and compositions thereforPublication Number: WO-2023225005-A1Priority Date: 2022-05-17
- Treatment of cancer with menin inhibitors and immuno-oncology agentsPublication Number: WO-2023172925-A1Priority Date: 2022-03-08
- Treatment of hematological malignancies with menin inhibitors and p-glycoprotein inhibitorsPublication Number: WO-2023150635-A1Priority Date: 2022-02-04
- Crystalline forms of N[4[4-(4-Morpholinyl)-7H-Pyrrolo[2-3-D]Pyrimidin-6-yl]Phenyl]-4-[[3(R)-[(1-Oxo-2-Protein-1-yl)Amino]-1-Piperidinyl]Methyl]2-Pyridinecarboxamide]Publication Number: US-12215113-B2Priority Date: 2023-01-18Grant Date: 2025-02-04
- CRYSTALLINE FORMS OF N-[4-[4-(4-MORPHOLINYL)-7H-PYRROLO[2,3-d]PYRIMIDIN-6-YL]PHENYL]-4-[[3(R)-[(1-OXO-2-PROPEN-1-YL)AMINO]-1-PIPERIDINYL]METHYL]-2-PYRIDINECARBOXAMIDE AS IRREVERSIBLE INHIBITORS OF MENIN-MLL INTERACTIONPublication Number: US-2024417404-A1Priority Date: 2023-01-18
- Crystalline forms of n-[4-[4-(4-morpholinyl)-7h-pyrrolo[2,3-d]pyrimidin-6- yl]phenyl]-4-[[3(r)-[(l-oxo-2-propen-l-yl)amino]-l-piperidinyl]methyl]-2-pyridinecarboxamide as a covalent inhibitor of menin-mll interactionPublication Number: WO-2024155710-A1Priority Date: 2023-01-18
- Crystalline forms of n-[4-[4-(4-morpholinyl)-7h-pyrrolo[2,3-d]pyrimidin-6-yl]phenyl]-4-[[3(r)-[(l-oxo-2-propen-l-yl)amino]-l-piperidinyl]methyl]-2- pyridinecarboxamide as a covalentinhibitor of menin-mll interactionPublication Number: WO-2024155719-A1Priority Date: 2023-01-18
- Combinations of lsd1 inhibitors and menin inhibitors for treating cancerPublication Number: WO-2024110649-A1Priority Date: 2022-11-24



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Rodriguez, Jose E.; Abitbol, Alexander; Abuzgaya, Fathi; Perez, Cesar; Mourya, Sanchita; Munneke, Brian; Morris, Stephan W.; Butler, Thomas (20 June 2023). “91-LB: COVALENT-111, a Phase 1/2 Trial of BMF-219, a Covalent Menin Inhibitor, in Patients with Type 2 Diabetes Mellitus—Preliminary Results”. Diabetes. 72 (Supplement_1) 91-LB. doi:10.2337/db23-91-LB. S2CID 259444592.
- Ravandi-Kashani, F.; Kishtagari, A.; Carraway, H.; Schiller, G.; Curran, E.; Yadav, B.; Cacovean, A.; Morris, S.; Butler, T.; Lancet, J. (23 June 2022). “P587: Covalent-101: A Phase 1 Study of BMF-219, A Novel Oral Irreversible Menin Inhibitor, in Patients with Relapsed/Refractory Acute Leukemia, Diffuse Large B-Cell Lymphoma, and Multiple Myeloma”. HemaSphere. 6: 486–487. doi:10.1097/01.HS9.0000845236.32931.83.
- Somanath, Priyanka; Lu, Daniel; Law, Brian; Archer, Tenley C.; Cacovean, Alexandru; Palmer, James T.; Kinoshita, Taisei; Butler, Thomas (5 November 2021). “Novel Irreversible Menin Inhibitor, BMF-219, Shows Potent Single Agent Activity in Clinically Relevant DLBCL Cells”. Blood. 138 (Supplement 1): 4318. doi:10.1182/blood-2021-148045.
| Clinical data | |
|---|---|
| Other names | BMF-219 |
| Legal status | |
| Legal status | Investigational |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2448172-22-1 |
| PubChem CID | 154988914 |
| ChemSpider | 115037287 |
| UNII | 2Z737MY35A |
| Chemical and physical data | |
| Formula | C31H34N8O3 |
| Molar mass | 566.666 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
/////////Icovamenib, antineoplastic, BMF-219, BMF 219, 2Z737MY35A, Menin-MLL inhibitor 21














