Efforts toward route evaluation and process optimization for the preparation of brexpiprazole (1) are described. Starting from commercially available dihydroquinolinone 11, a three-step synthesis route composed of O-alkylation, oxidation, and N-alkylation was selected for industry-oriented process development aiming to reduce side reactions and achieve better impurity profiles. The reaction conditions of the three steps were investigated, and the control strategy for the process-related impurities was established. The optimized process was validated on the kilogram scale and now is viable for commercialization, with the results of not less than 99.90% purity of 1 (by HPLC) and not more than 0.05% of persistent impurities 15 and 16
Industry-Oriented Route Evaluation and Process Optimization for the Preparation of Brexpiprazole
†Key Laboratory of Plant Resources and Chemistry in Arid Regions, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, South Beijing Road 40−1, Urumqi, Xinjiang 830011, P. R. China
‡University of Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, P. R. China
§CAS Key Laboratory for Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai 201203, P. R. China
∥Topharman Shanghai Co., Ltd., Building 1, No. 388 Jialilue Road, Zhangjiang Hitech Park, Shanghai 201209, P. R. China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00438
ESI-MS: m/z = 434.22 [M + H].
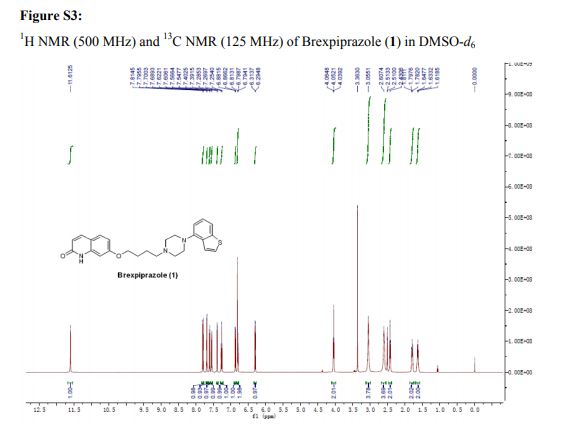
1H NMR (500 MHz, DMSO-d6) δ (ppm): 11.61 (s, 1H), 7.80 (d, J = 9.4 Hz, 1H), 7.69 (d, J = 5.5 Hz, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.56 (d, J = 9.4 Hz, 1H), 7.40 (d, J = 5.5 Hz, 1H), 7.27 (d, J = 7.8 Hz, 1H), 6.87 (d, J = 7.6 Hz, 1H), 6.84–6.78 (m, 2H), 6.30 (d, J = 9.4 Hz, 1H), 4.05 (t, J = 6.4 Hz, 2H), 3.06 (brs, 4H), 2.61 (brs, 4H), 2.43 (t, J = 7.1 Hz, 2H), 1.86–1.75 (m, 2H), 1.69–1.57 (m, 2H).
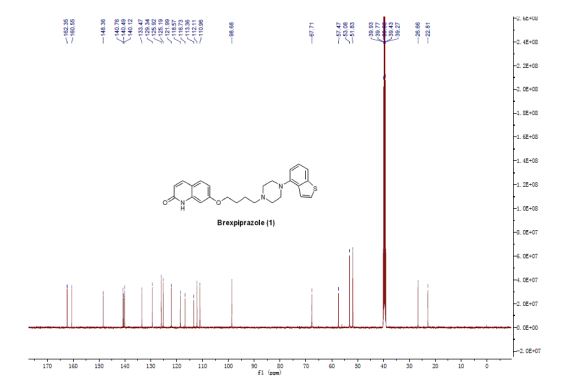
13C NMR (125 MHz, DMSO-d6) δ (ppm): 162.35, 160.55, 148.36, 140.76, 140.49, 140.12, 133.47, 129.34, 125.92, 125.19, 121.99, 118.57, 116.73, 113.36, 112.11, 110.96, 98.68, 67.71, 57.47, 53.08, 51.83, 26.66, 22.81.
/////////////Brexpiprazole


















