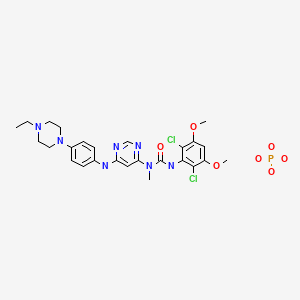
Infigratinib phosphate
FDA APPR Truseltiq 2021/5/28
インフィグラチニブリン酸塩;
3-(2,6-dichloro-3,5-dimethoxyphenyl)-1-[6-[4-(4-ethylpiperazin-1-yl)anilino]pyrimidin-4-yl]-1-methylurea;phosphoric acid
- BGJ 398
- BGJ-398
- BGJ398
- NVP-BGJ398
- WHO 10032
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Infigratinib acetate | 03D0789NYP | 1310746-17-8 | XHCQHOGMMJKLRU-UHFFFAOYSA-N |
| Infigratinib hydrochloride | WY8VD4RV77 | 1310746-15-6 | VBAIJSJSFCXDJB-UHFFFAOYSA-N |
| Infigratinib mesylate | E223Z0KWCC | 1310746-12-3 | BXJJFNXYWJLBOS-UHFFFAOYSA-N |
| Infigratinib phosphate | 58BH47BV6S | 1310746-10-1 | GUQNHCGYHLSITB-UHFFFAOYSA-N |
International/Other BrandsTruseltiq (BridgeBio Pharma, Inc.)
| Formula |
C26H31Cl2N7O3. H3PO4
|
|---|---|
| CAS |
1310746-10-1
FREE form 872511-34-7
|
| Mol weight |
658.4706
|
- Originator Novartis
- Developer Array BioPharma; Novartis; Novartis Oncology; QED Therapeutics
- Class Aniline compounds; Antineoplastics; Chlorobenzenes; Methylurea compounds; Phenyl ethers; Piperazines; Pyrimidines; Small molecules
- Mechanism of Action Type 1 fibroblast growth factor receptor antagonists; Type 3 fibroblast growth factor receptor antagonists; Type 4 fibroblast growth factor receptor antagonists; Type-2 fibroblast growth factor receptor antagonists
- Orphan Drug Status Yes – Cholangiocarcinoma
- RegisteredCholangiocarcinoma
- Phase IIIBladder cancer; Urogenital cancer
- Phase IIAchondroplasia; Head and neck cancer
- Phase IBreast cancer
- Phase 0Glioblastoma
- DiscontinuedHaematological malignancies; Malignant melanoma; Solid tumours
- 31 May 2021Clinical development is ongoing in Bladder cancer (QED Therapeutics pipeline, May 2020)
- 28 May 2021Registered for Cholangiocarcinoma (Second-line therapy or greater, Metastatic disease, Inoperable/Unresectable, Late-stage disease) in USA (PO) – First global approval (under Project Orbis using RTOR program)
- 28 May 2021Efficacy and safety data from a phase II trial in Cholangiocarcinoma released by QED Therapeutics
Infigratinib, sold under the brand name Truseltiq, is an anti-cancer medication used to treat cholangiocarcinoma (bile duct cancer).[1][2]
Infigratinib is a receptor tyrosine kinase inhibitor (and more specifically an inhibitor of the fibroblast growth factor receptors FGFR1, FGFR2, FGFR3).[3][1][2] It was designated an orphan drug by the U.S. Food and Drug Administration (FDA) in 2019,[4] and it was approved for medical use in the United States in May 2021.[2]
Infigratinib is a pan-fibroblast growth factor receptor (FGFR) kinase inhibitor. By inhibiting the FGFR pathway, which is often aberrated in cancers such as cholangiocarcinoma, infigratinib suppresses tumour growth.1 Cholangiocarcinoma is the most common primary malignancy affecting the biliary tract and the second most common primary hepatic malignancy.2 Infitratinib is a pan-FGFR inhibitor, as it is an ATP-competitive inhibitor of all four FGFR receptor subtypes.1
On May 28, 2021, the FDA granted accelerated approval to infigratinib – under the market name Truseltiq – for the treatment of previously treated, unresectable locally advanced or metastatic cholangiocarcinoma in adults with a fibroblast growth factor receptor 2 (FGFR2) fusion or another rearrangement as detected by an FDA-approved test.5 This approval follows pemigatinib, another FGFR inhibitor approved by the FDA for the same therapeutic indication.
Infigratinib is indicated for the treatment of previously treated, unresectable locally advanced or metastatic cholangiocarcinoma in adults with a fibroblast growth factor receptor 2 (FGFR2) fusion or another rearrangement as detected by an FDA-approved test.4
Medical uses
Infigratinib is indicated for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma (bile duct cancer) with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test.[1]
PAPER
Journal of Medicinal Chemistry (2011), 54(20), 7066-7083.
https://pubs.acs.org/doi/10.1021/jm2006222

A novel series of N-aryl-N′-pyrimidin-4-yl ureas has been optimized to afford potent and selective inhibitors of the fibroblast growth factor receptor tyrosine kinases 1, 2, and 3 by rationally designing the substitution pattern of the aryl ring. On the basis of its in vitro profile, compound 1h (NVP-BGJ398) was selected for in vivo evaluation and showed significant antitumor activity in RT112 bladder cancer xenografts models overexpressing wild-type FGFR3. These results support the potential therapeutic use of 1h as a new anticancer agent.
PATENT
US 9067896
PATENT
WO 2020243442
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020243442
In 2018, it was estimated that 150,350 new patients would be diagnosed with urinary system cancer: 81,190 urinary bladder; 65,340 kidney and renal pelvis; and, 3,820 ureter and other urinary organs. Excluding non-urothelial kidney cancers, approximately 5 to 10% of all urothelial carcinomas are upper tract urothelial carcinomas (UTUC). The incidence of UTUC is 2 to 3 times greater in men than women (Siegel et al, 2018; Roupret et al, 2015).
[0003] In contrast to invasive urinary bladder cancer (UCB), UTUC has a more aggressive clinical course. At the time of diagnosis, 60% of patients with UTUC have invasive cancer compared to 15% to 25% of patients with UCB (Roupret et al, 2015; Margulis et al., 2009). Thirty-six percent have regional disease and 9% distant disease (Raman et al., 2010). A large retrospective review of 1363 patients with UTUC who underwent radical nephroureterectomy (RNU) at 12 centers demonstrated that 28% of the total population had recurrence after RNU (Margulis et al, 2009).
[0004] To reduce the morbidity and mortality in patients with UTUC, neoadjuvant or adjuvant treatment is needed. The POUT study, a large randomized trial in UTUC supports the use of standard-of-care adjuvant cisplatin-based chemotherapy (Birtle et al., 2020). Because many patients with UTUC will have one remaining kidney following RNU and frequently have other significant co-morbid conditions, cisplatin-based therapy is not well tolerated (NCCN Guidelines Version 3, 2018). Renal function before and after RNU greatly limits the number of patients with UTUC who are eligible for platinum-based neoadjuvant or adjuvant therapy. Therefore, targeted therapies are needed for treating UTUC (Lane et al., 2010).
[0005] Despite demonstrated survival benefit for neoadjuvant treatment of invasive UCB, many patients with invasive UCB are unlikely to receive (neo)adjuvant cisplatin-based chemotherapy, due in part to cisplatin ineligibility (Porter et al., 2011). In addition, residual disease following neoadjuvant therapy is associated with a poor prognosis (Grossman et al, 2003). Therefore,
there remains an unmet need for a substantial proportion of patients with invasive UCB who are ineligible or refuse to receive cisplatin-based adjuvant chemotherapy or who have residual disease following neoadjuvant therapy.
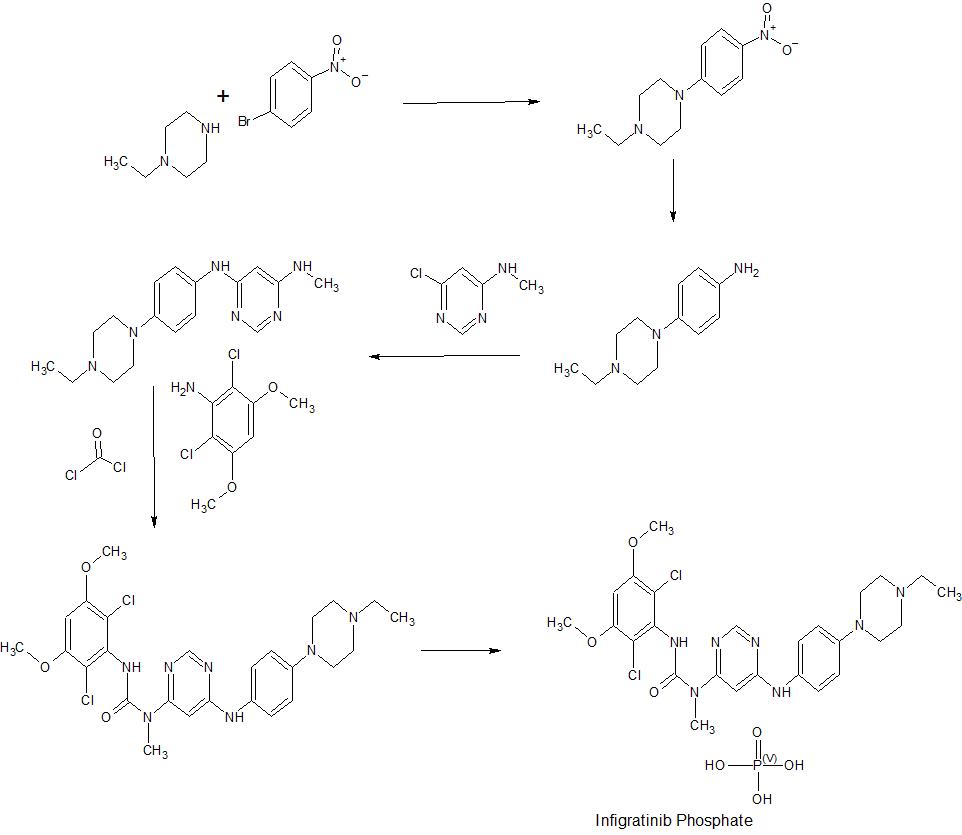
Infigratinib, as depicted in formula (I), is a selective and ATP-competitive pan-fibroblast growth factor receptor (FGFR) kinase inhibitor, also known as 3-(2,6-dichloro-3,5-dimethoxyphenyl)- 1 – { 6- [4-(4-ethyl- 1 -piperazin- 1 -yljphenylamino] -pyrimidinyl-4-yl } – 1 -methylurea. Infigratinib selectively inhibits the kinase activity of FGFR1, FGFR2, FGFR3, and
FGFR4.
PATENT
WO 2011071821
https://patents.google.com/patent/WO2011071821A1/en
References
- ^ Jump up to:a b c d “Infigratinib prescribing information” (PDF). U.S. Food and Drug Administration. May 2021.
- ^ Jump up to:a b c “BridgeBio Pharma’s Affiliate QED Therapeutics and Partner Helsinn Group Announce FDA Approval of Truseltiq (infigratinib) for Patients with Cholangiocarcinoma” (Press release). BridgeBio Pharma. 28 May 2021. Retrieved 28 May 2021 – via GlobeNewswire.
- ^ Botrus G, Raman P, Oliver T, Bekaii-Saab T (April 2021). “Infigratinib (BGJ398): an investigational agent for the treatment of FGFR-altered intrahepatic cholangiocarcinoma”. Expert Opinion on Investigational Drugs. 30 (4): 309–316. doi:10.1080/13543784.2021.1864320. PMID 33307867.
- ^ “Infigratinib Orphan Drug Designations and Approvals”. U.S. Food and Drug Administration (FDA). 11 September 2019. Retrieved 30 May 2021.
External links
- “Infigratinib”. Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02150967 for “A Phase II, Single Arm Study of BGJ398 in Patients With Advanced Cholangiocarcinoma” at ClinicalTrials.gov
| Efficacy |
Antineoplastic, Angiogenesis inhibitor
|
|---|---|
| Disease |
Cholangiocarcinoma (FGFR2 fusion or other rearrangement)
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Truseltiq |
| Other names | BGJ-398 |
| License data |
|
| Routes of administration |
By mouth |
| Drug class | Tyrosine kinase inhibitor |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H31Cl2N7O3 |
| Molar mass | 560.48 g·mol−1 |
| 3D model (JSmol) | |
|
|
|
////////Infigratinib phosphate, FDA 2021 APPROVALS 2021, Truseltiq, インフィグラチニブリン酸塩 , Orphan Drug, Cholangiocarcinoma, BGJ 398, BGJ-398, BGJ398, NVP-BGJ398, WHO 10032