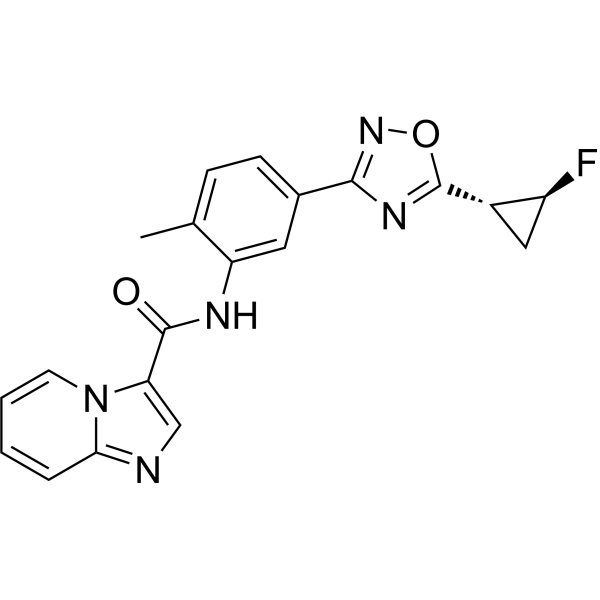
Labuxtinib
CAS 1426449-01-5
Labuxtinib, also known as EVT-8565072,
| Molecular Weight | 377.37 |
|---|---|
| Formula | C20H16FN5O2 |
- N-[5-[5-[(1R,2S)-2-fluorocyclopropyl]-1,2,4-oxadiazol-3-yl]-2-methylphenyl]imidazo[1,2-a]pyridine-3-carboxamide
- N-(5-(5-((1R,2S)-2-fluorocyclopropyl)-1,2,4-oxadiazol-3-yl)-2-methylphenyl)imidazo[1,2-a]pyridine-3-carboxamide
- QNX4G754W6
Labuxtinib is c-kit tyrosine kinase inhibitor.
Labuxtinib, also known as EVT-8565072, is a synthetic organic compound that acts as a tyrosine kinase inhibitor, specifically targeting c-KIT. It is a potential anti-cancer agent and is likely the INN (Proposed International Nonproprietary Name) for Third Harmonic Bio‘s candidate KIT inhibitor, THB335. The initial clinical lead, THB001, was discontinued due to hepatotoxicity, and THB335 is a follow-up molecule with structural modifications to address this issue.
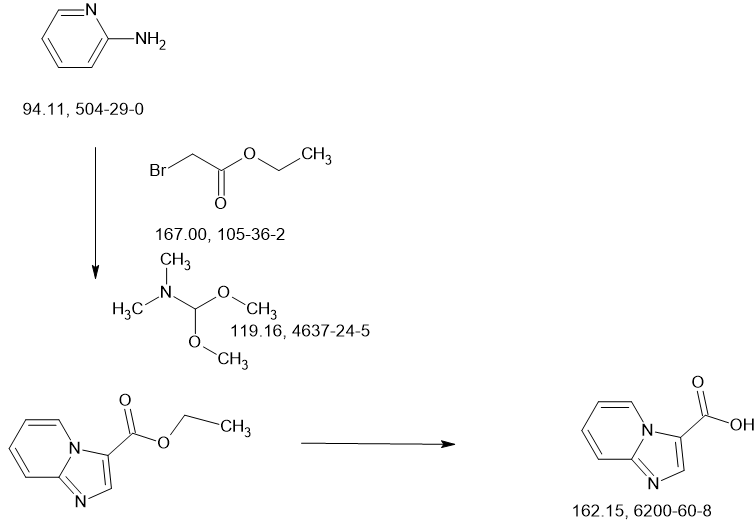
SCHEME
SIDECHAIN
MAIN
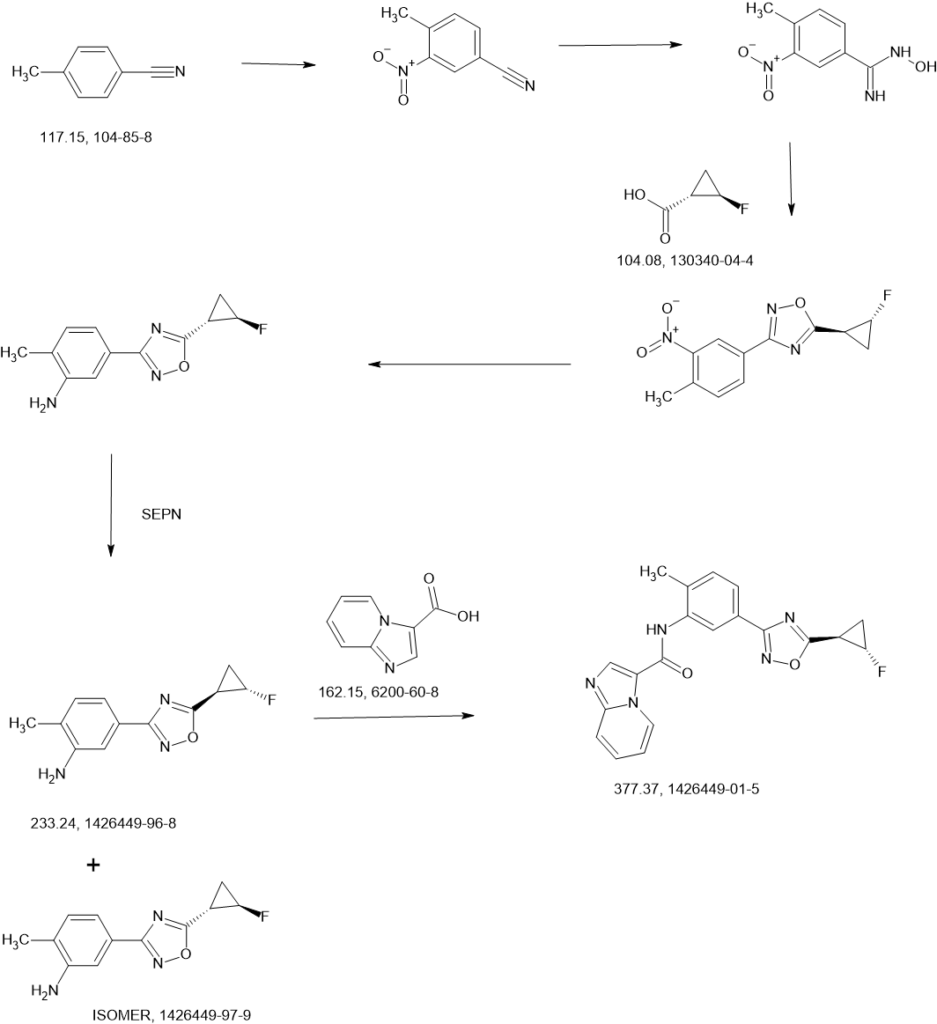
WO2013033070 F91
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013033070&_cid=P11-MBX2OL-77562-1
PATENT
WO2022109595 COMPD 1
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022109595&_cid=P11-MBX2U2-81593-1
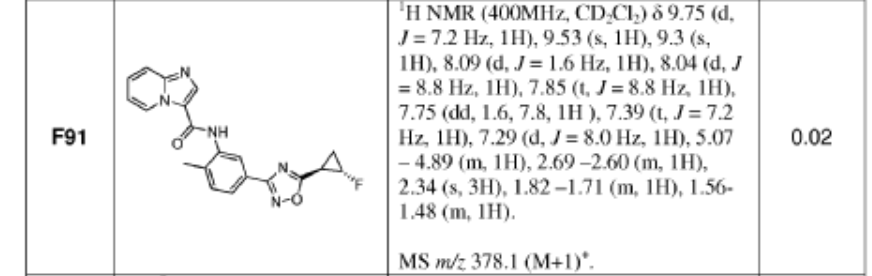
A. Compound 1
[0022] As defined above, a pharmaceutical composition of the present invention is a micronized powder comprising Compound 1. Compound 1 can be prepared according to example Fl 10 of WO 2013/033070 Al, which is incorporated by reference herein, as summarized in the
Scheme 1 provided below:
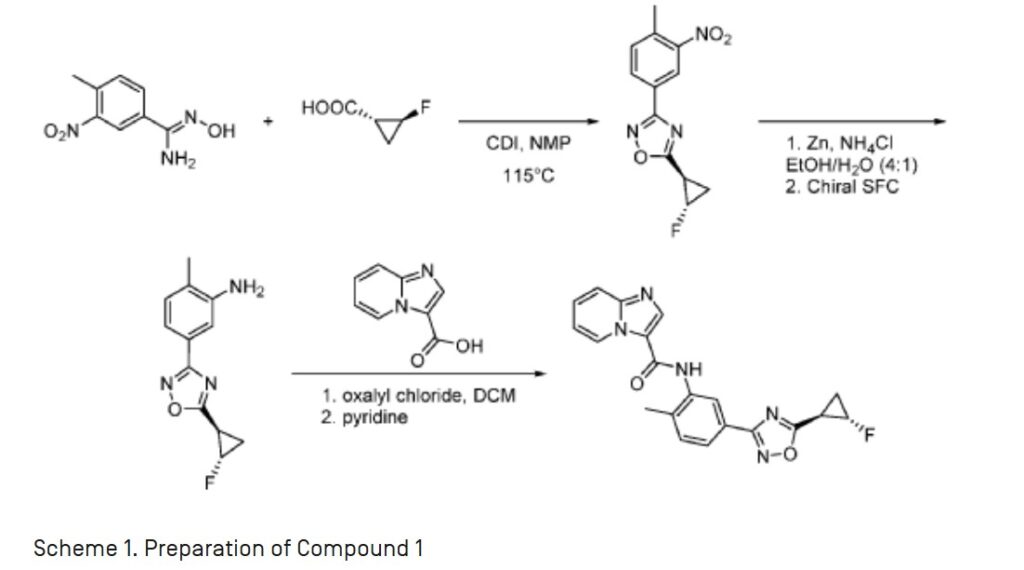
Scheme 1. Preparation of Compound 1
[0023] In some embodiments, the pharmaceutical composition is a micronized powder comprising dry microparticles of Compound 1. In some embodiments the microparticles of Compound 1 comprise amorphous Compound 1. In some embodiments, the microparticles of Compound 1 comprise a crystalline solid form of Compound 1. In some embodiments, the microparticles of Compound 1 comprise a crystalline free base solid form of Compound 1. In some embodiments, the microparticles of Compound 1 comprise a crystalline salt solid form of Compound 1.
[0024] In some embodiments, the crystalline solid form of Compound 1 is an anhydrate form. In some embodiments, the crystalline solid form of Compound 1 is a hydrate form. In some embodiments, the crystalline solid form of Compound l is a monohydrate. In some embodiments, the crystalline solid form of Compound l is a hemihydrate. In some embodiments, the crystalline solid form of Compound 1 is a dihydrate.
[0025] In some embodiments, the microparticles of Compound 1 comprise a crystalline solid form of Compound 1 disclosed in PCT/CN2020/090060, which is incorporated by reference herein.
PATENT
PATENT
WO2020228746 NOVARTIS
WO2013033070 IRM LLC
As of January 2025, Labuxtinib remains in preclinical/early clinical development, with no publicly disclosed Phase 1 data . THB’s strategy focuses on:
- Targeting KIT-driven malignancies: GISTs, mastocytosis, melanoma
- Addressing resistance: Overcoming mutations conferring resistance to imatinib/nilotinib .
///////////Labuxtinib, QNX4G754W6, EVT-8565072, EVT 8565072














