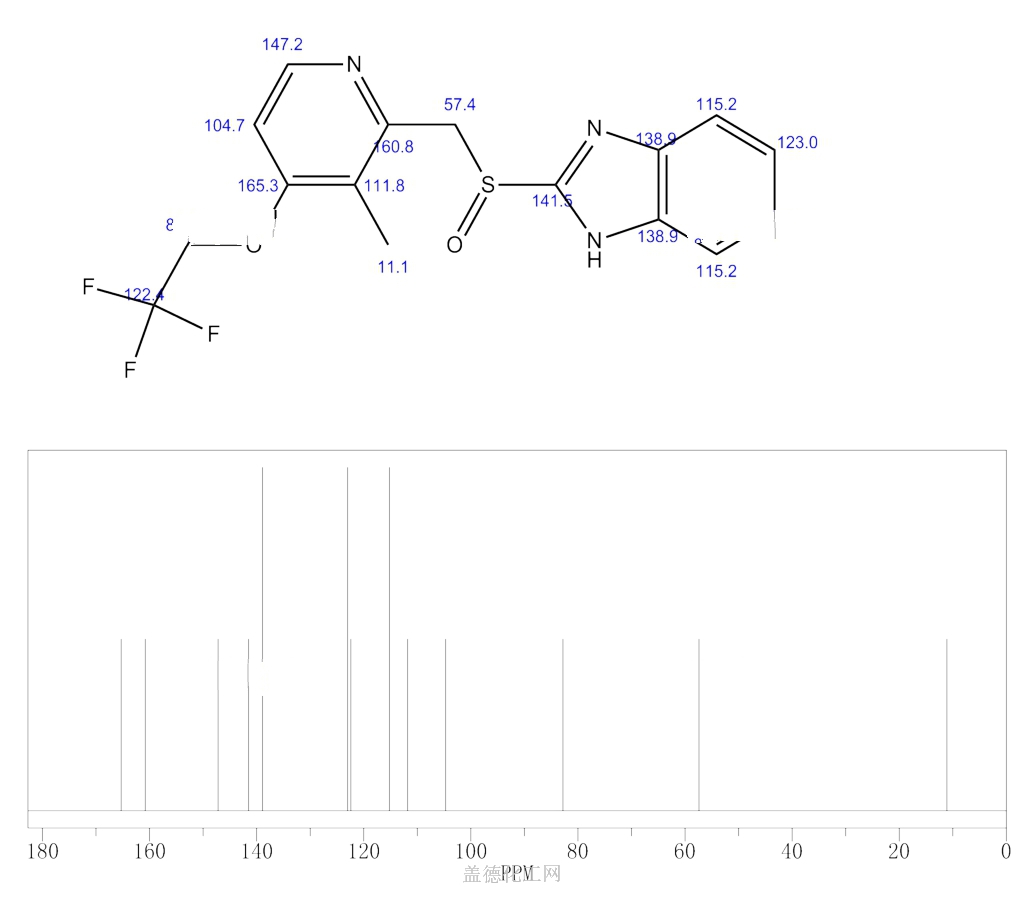
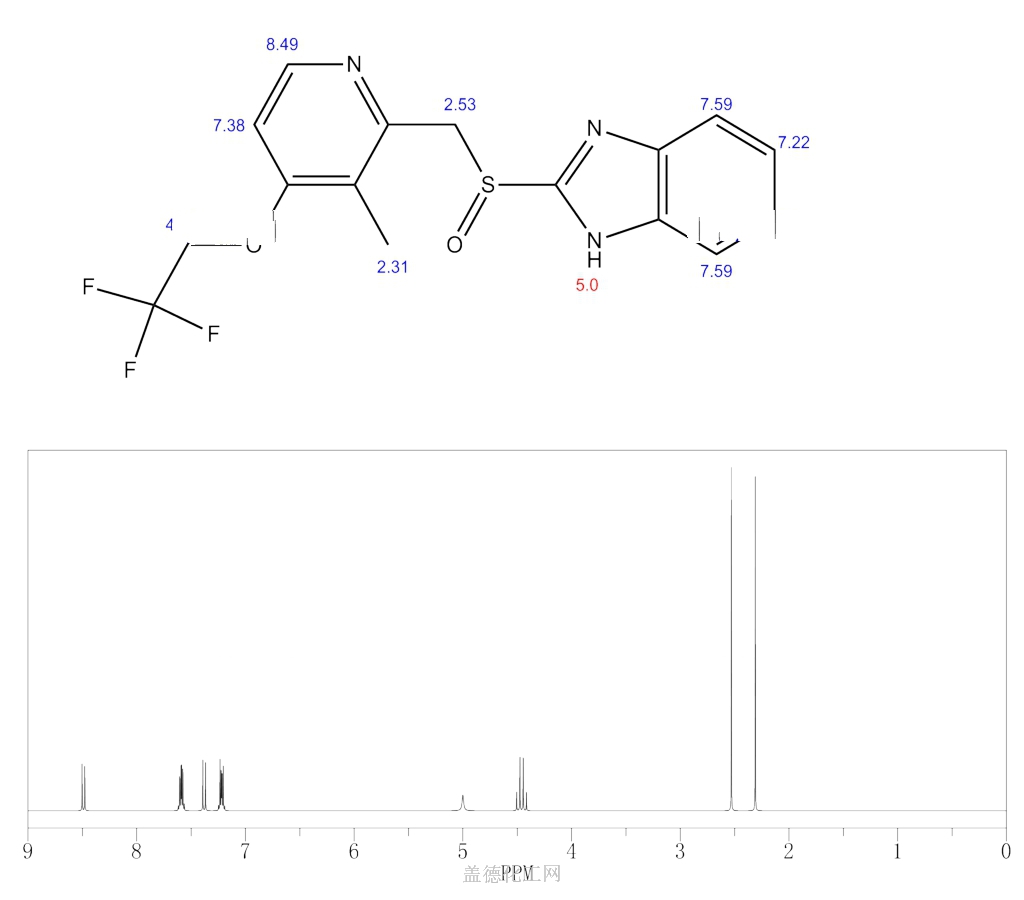
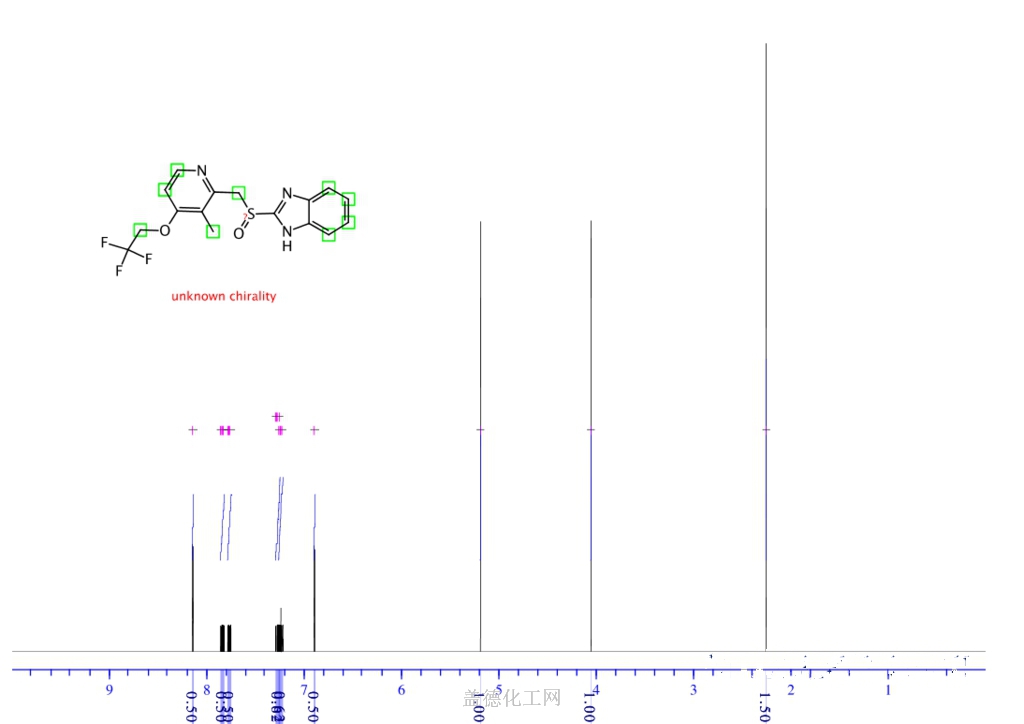
- Molecular FormulaC16H14F3N3O2S
- Average mass369.362 Da
Lansoprazole, AG-1749, ABT-006, CG-4801, A-65006, Ogast, Lanzor, Lanzo, Agopton, Opiren, Bamalite, Takepron, Lansox, Lansox, Ogastro, Monolitum, Prevacid, Zoton
Lansoprazole, sold under the brand name Selanz SR among others, is a medication which reduces stomach acid.[2] It is used to treat peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome.[3] Effectiveness is similar to other proton pump inhibitors (PPIs).[4] It is taken by mouth.[2] Onset is over a few hours and effects last up to a couple of days.[2]
Common side effects include constipation, abdominal pain, and nausea.[2][5] Serious side effects may include osteoporosis, low blood magnesium, Clostridium difficile infection, and pneumonia.[2][5] Use in pregnancy and breastfeeding is of unclear safety.[1] It works by blocking H+/K+-ATPase in the parietal cells of the stomach.[2]
Lansoprazole was patented in 1984 and came into medical use in 1992.[6] It is available as a generic medication.[3] In 2017, it was the 188th most commonly prescribed medication in the United States, with more than three million prescriptions.[7][8]
Medical uses
Lansoprazole is used for treatment of:[5]
- Ulcers of the stomach and duodenum, and NSAID-induced ulcers
- Helicobacter pylori infection, alongside antibiotics (adjunctive treatment), treatment to kill H. pylori causing ulcers or other problems involves using two other drugs besides lansoprazole known as “triple therapy“, and involves taking twice daily for 10 or 14 days lansoprazole, amoxicillin, and clarithromycin
- Gastroesophageal reflux disease
- Zollinger-Ellison syndrome[9]
There is no good evidence that it works better than other PPIs.[4]
Side effects
Side effects of PPIs in general[10] and lansoprazole in particular[11] may include:[5]
- Common: diarrhea, abdominal pain[12]
- Infrequent: dry mouth, insomnia, drowsiness, blurred vision, rash, pruritus
- Rarely and very rarely: taste disturbance, liver dysfunction, peripheral oedema, hypersensitivity reactions (including bronchospasm, urinary, angioedema, anaphylaxis), photosensitivity, fever, sweating, depression, interstitial nephritis, blood disorders (including leukopenia, leukocytosis, pancytopenia, thrombocytopenia), arthralgia, myalgia, skin reactions[13] including (erythroderma[14] Stevens–Johnson syndrome, toxic epidermal necrolysis, bullous eruption)
PPIs may be associated with a greater risk of hip fractures and Clostridium difficile-associated diarrhea.[5]: 22
Interactions
Lansoprazole interacts with several other drugs, either due to its own nature or as a PPI.[15]
- PPIs reduce absorption of antifungals (itraconazole and ketoconazole) [16] and possibly increase digoxin in plasma
- Increases plasma concentrations of cilostazol (risk of toxicity)
Lansoprazole possibly interacts with, among other drugs:
- sucralfate
- ampicillin
- bisacodyl
- clopidogrel
- delavirdine
- fluvoxamine
- iron salts
- voriconazole
- aminophylline and theophylline
- astemizole
Chemistry
It is a racemic 1:1 mixture of the enantiomers dexlansoprazole and levolansoprazole.[17] Dexlansoprazole is an enantiomerically pure active ingredient of a commercial drug as a result of the enantiomeric shift. Lansoprazole’s plasma elimination half-life (1.5 h) is not proportional to the duration of the drug’s effects to the person (i.e. gastric acid suppression).[18]
History
Lansoprazole , available in the name of Selanz SR, was originally synthesized at Takeda and was given the development name AG 1749.[19] Takeda patented it in 1984 and the drug launched in 1991.[20] In the United States, it was approved for medical use in 1995.[21]
Society and culture
Patents
The lansoprazole molecule is off-patent and so generic drugs are available under many brand names in many countries;[22] there are patents covering some formulations in effect as of 2015.[23] Patent protection expired on 10 November 2009.[24][25]
Availability
Since 2009, lansoprazole has been available over the counter (OTC) in the U.S. as Prevacid 24HR[26][27] and as Lansoprazole 24HR.[28] In Australia, it is marketed by Pfizer as Zoton.[citation needed]
Research
In vitro experiments have shown that lansoprazole binds to the pathogenic form of tau protein.[29] As of 2015 laboratory studies were underway on analogs of lansoprazole to explore their use as potential PET imaging agents for diagnosing tauopathies including Alzheimer’s disease.[29]
SYN
English: doi: 10.1248/cpb.38.2853
SYN
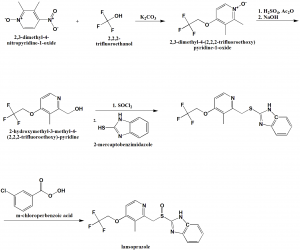
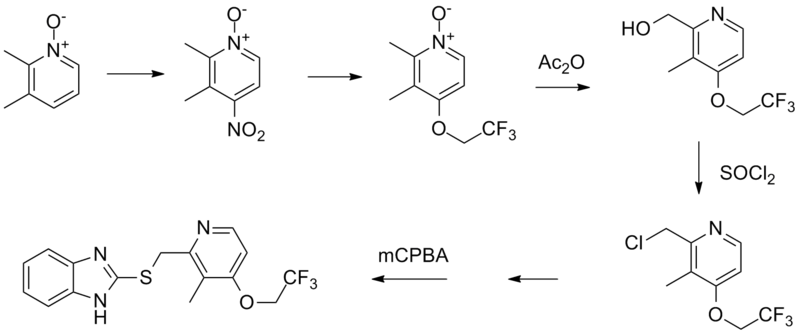
Method of synthesis
i. 2,3-dimethyl-4-nitropyridine-1-oxide is reacted with 2,2,2-trifluoroethanol in presence of potassium carbonate to give 2,3-dimethyl-4-(2,2,2-trifluoro-ethoxy)pyridine-1-oxide.
ii. The compound so formed is treated with acetic anhydride in acidic conditions followed by nutrilizing with sodium hydroxide solution to get 2-hydroxymethyl-3-methyl-4-(2,2,2-trifluoroethoxy)-pyridine
iii. Last is treated with thionyl chloride followed by reaction with 2-mercaptobenzimidazole to get 2-[3-methyl-4-(2,2,2-trifluoroethoxy)pyrid-2-ylmethylthio]benzimidazole.
iv. Above formed compound is reacted with m-chloro-perbenzoic acid to get lansoprazole.[2]
SYN
Proton Pump Inhibitors
Ruben Vardanyan, Victor Hruby, in Synthesis of Best-Seller Drugs, 2016
Lansoprazole–Prevacid
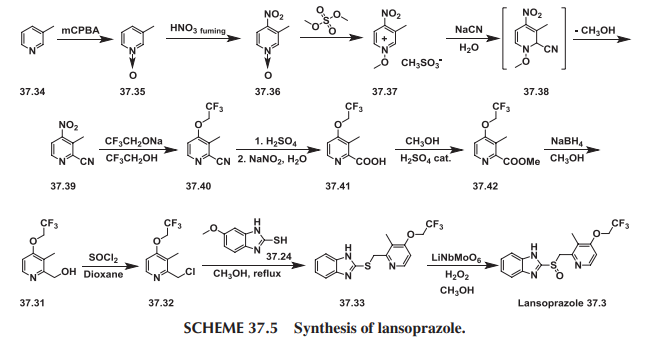
Lansoprazole (37.3) is the second approved gastric acid pump inhibitor. The common approach for the synthesis of lansoprazole involves coupling of mercapto-benzimidazole (37.24) with a new 2-chloromethylpyridine derivative (37.32) followed by oxidation of the prochiral sulfide group with m-chloroperbenzoic acid or hydrogen peroxide was first disclosed by Nohara and Maki [73], with followed improvements in patents [74-78] and briefly summed up in papers [79-80].
Lansoprazole synthesis is represented on the Scheme 37.4.


Scheme 37.4. Synthesis of lansoprazole.
In principle it repeats the synthesis Scheme of omeprazole, differing in details and characteristics, for example, in place of 2,3,5-collidine (37.15) as a starting material, 2,3-lutidine (37.27) was selected, and the methoxy group in the fourth position of pyridine ring was replaced by the 2,2,2-trifluoroethoxy group.
Another interesting approach has been demonstrated [81]. In this case, 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine (37.32) was prepared starting with 3-picoline (37.34), which was oxidized using peracids (i.e., m-chloroperoxybenzoic acid) to produce 3-methylpyridineN-oxide (37.35). The obtained product was nitrated with fuming nitric acid to produce 3-methyl-4-nitropyridine N-oxide (37.36). The prepared N-oxide was treated with dimethylsulfate at 65 to 70°C to form N-methoxypyridinium salt (37.37), the aqueous solution of which on cooling was treated with sodium cyanide to produce an after formation of intermediate (37.38) and elimination of methanol 2-cyano-3-methyl-4-nitropyridine (37.39). This method for the synthesis of 2-cyanopyridines via addition of cyanide ion to N-alkoxy-quaternary salts of pyridines, supplements the plethora of Reissert-Kaufmann reactions in the quinoline and isoquinoline series previously described [82]. The nitro group in (37.39) was replaced by the 2,2,2-trifluoroethoxy group by a direct reaction with sodium trifluoroethoxide in trifluoroethanol that produced ether (37.40). The next step—transformation of nitrile group in prepared 2-cyanopyridine (37.40) to 2-carboxypyridine (37.41)—was carried out in a one-pot procedure by heating the 2-cyano compound in the presence of concentrated sulfuric acid followed by reaction of the intermediate amide with sodium nitrite under aqueous acidic conditions [83,84]. The obtained acid was esterified in methanol with a catalytic amount of sulfuric acid to produce ester (37.42). The ester (37.42) was reduced by NaBH4, producing the above-described 2-hydroxymethyl- pyridine derivative (37.31) followed by a reaction with thionyl chloride in dioxane that produced the required 2-chloromethylpyridine compound (37.32). Direct reaction of the last with 2-mercaptobenzimidazole (37.34) in methanol, even without use of any base, produced a sulfide (37.33) in high yield. The oxidation of the last to lansoprazole (37.3) has been carried out by various oxidants and catalysts, which, together with the desired sulfoxide, produced a certain amount of overoxidized product. Oxidizing sulfide (37.33) with a new oxidation method made up of the use of the composite metal oxide catalyst, LiNbMoO6, in methanol and 35% H2O2 as an oxidant sulfide (37.33) was successfully oxidized to desired lansoprazole (37.3) (Scheme 37.5.).


Scheme 37.5. Synthesis of lansoprazole.
Lansoprazole is the second inhibitor of the gastric H+/K+-ATPase to be marketed for the treatment of peptic ulcer disease and reflux esophagitis, erosive esophagitis, and Zollinger-Ellison syndrome. It is an inhibitor of gastric acid secretion and also exhibits antibacterial activity against H. pylori in vitro. More common side effects of lansoprazole are diarrhea and skin rash or itching. Less-common side effects are abdominal pain, joint pain, nausea, vomiting, and increased or decreased appetite [85-91].
SYN
| AU 8545895; EP 0174726; ES 8607288; JP 1986050978; US 4628098; US 4689333 |
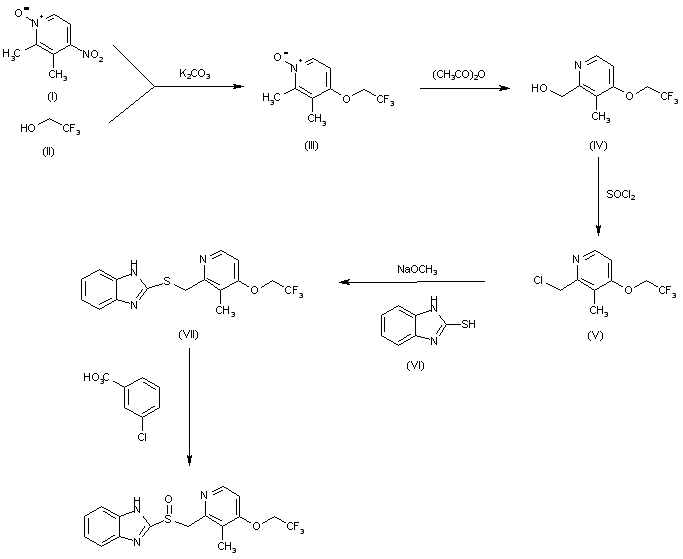
The condensation of 2,3-dimethyl-4-nitropyridine N-oxide (I) with 2,2,2-trifluoroethanol (II) by means of K2CO3 in hot HMPT gives 2,3-dimethyl-4-(2,2,2-trifluoroethoxy)pyridine N-oxide (III), which by isomerization in acetic anhydride at 100 C is converted to 2-(hydroxymethyl)-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine (IV). The reaction of (IV) with SOCl2 in refluxing CHCl3 affords the corresponding chloromethyl derivative (V), which is condensed with 2-mercaptobenzimidazole (VI) by means of sodium methoxide in refluxing methanol to yield 2-(2-benzimidazolylthiomethyl)-3-methyl-4-(2,2,2-trifluoroethoxy)pyridin (VII). Finally, this compound is oxidized with m-chloroperbenzoic acid in CHCl3.
SYN
SYN
Chemical Synthesis
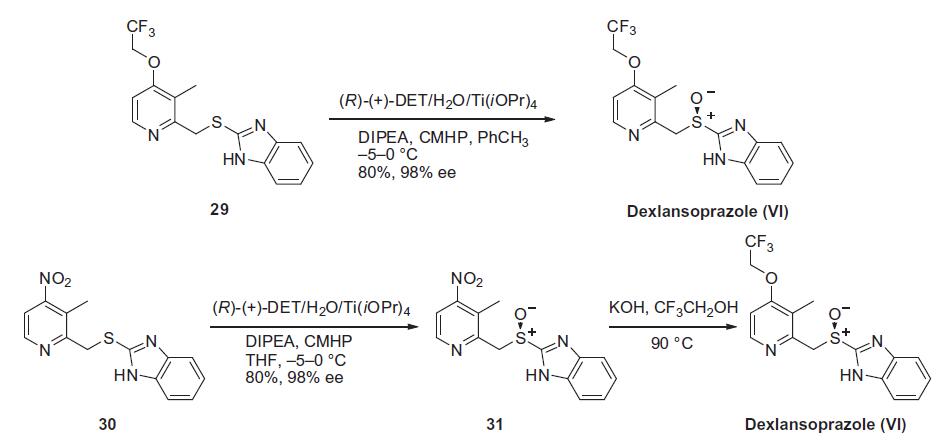
Similar to the synthesis of the chiral sulfoxide of armodafinil vide supra, the preparation of the chiral sulfoxide of lansoprazole utilized the catalytic oxidation method developed by Kagan and co-workers (the Scheme). Two routes have been reported that describe the preparation of dexlansoprazole on large scale. The first route developed by Takeda reacts commercially available thioether 29, also used to make lansoprazole, under the Kagan asymmetric oxidation conditions and the alternative route utilizes the cheaper commercial intermediate nitrosulfide 30 in the analogous asymmetric oxidation by Kagan). Thus, the catalyst complex consisting of (+)-DET, Ti(OiPr)4 and water was formed in the presence of thioether 29 in toluene at 30–40°C. The reaction mixture was then cooled to 5 °C and DIPEA and cumene hydroperoxide (CMHP) were added to give, after aqueous work-up and in situ crystallization from the organic layer, dexlansoprazole (VI) in 98% ee. No yield was given in the patent. An alternate, but similar, sequence was also described wherein the nitrosulfide intermediate 30 was subjected to similar oxidative conditions that gave intermediate nitro compound 31 in 80% yield and 98% ee. Compound 31 was treated with KOH and trifluoroethanol to provide dexlansoprazole (VI).
R-(+)-Lansoprazole Preparation Products And Raw materials
PATENT
https://patents.google.com/patent/WO2008087665A2/en


join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
Literatures:
Chemical and Pharmaceutical Bulletin, , vol. 38, # 10 p. 2853 – 2858
Literatures:
KRKA, tovarna zdravil, d.d., Novo mesto Patent: EP2030973 A1, 2009 ; Location in patent: Page/Page column 11 ;
Literatures:
RECORDATI INDUSTRIA CHIMICA E FARMACEUTICA SPA Patent: WO2008/77866 A1, 2008 ; Location in patent: Page/Page column 16-17; 19 ;
Yield: ~92%
Patent
References
- ^ Jump up to:a b c “Lansoprazole Use During Pregnancy”. Drugs.com. Retrieved 3 March 2019.
- ^ Jump up to:a b c d e f “Lansoprazole Monograph for Professionals”. Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ Jump up to:a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 79–80. ISBN 9780857113382.
- ^ Jump up to:a b “[99] Comparative effectiveness of proton pump inhibitors | Therapeutics Initiative”. 28 June 2016. Retrieved 14 July 2016.
- ^ Jump up to:a b c d e “Lansoprazole capsule, delayed release pellets”. DailyMed. 11 October 2016. Retrieved 31 December 2019.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 445. ISBN 9783527607495.
- ^ “The Top 300 of 2020”. ClinCalc. Retrieved 11 April 2020.
- ^ “Lansoprazole – Drug Usage Statistics”. ClinCalc. Retrieved 11 April 2020.
- ^ Hirschowitz BI, Mohnen J, Shaw S (August 1996). “Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrome”. Aliment. Pharmacol. Ther. 10 (4): 507–22. doi:10.1046/j.1365-2036.1996.10152000.x. PMID 8853754. S2CID 10668517.
- ^ British National Formulary (Free registration required) 1.3.5 Proton pump inhibitors
- ^ British National Formulary (Free registration required) Lansoprazole
- ^ “Prevacid (Lansoprazole) Drug Information: Side Effects and Drug Interactions – Prescribing Information at RxList”. RxList. Retrieved 9 February 2016.
- ^ K C Singhal & S Z Rahman, Lansoprazole Induced Adverse Effects on the Skin, Indian Medical Gazette, July 2001, Vol. CXXXV. N0. 7: 223-225
- ^ Sterry W, Assaf C (2007). “Erythroderma”. In Bolognia JL (ed.). Dermatology. St. Louis: Mosby. p. 154. ISBN 978-1-4160-2999-1..
- ^ British National Formulary (Free registration required) Lansoprazole interactions
- ^ Piscitelli, S. C.; Goss, T. F.; Wilton, J. H.; d’Andrea, D. T.; Goldstein, H; Schentag, J. J. (1991). “Effects of ranitidine and sucralfate on ketoconazole bioavailability”. Antimicrobial Agents and Chemotherapy. 35 (9): 1765–1771. doi:10.1128/aac.35.9.1765. PMC 245265. PMID 1952845.
- ^ “Pharmacy Benefit Update”. Retrieved 2 July 2014.
- ^ “Prevacid Pharmacology, Pharmacokinetics, Studies, Metabolism”. RxList.com. 2007. Archived from the original on 16 August 2000. Retrieved 14 April 2007.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 102. ISBN 9783527607495.
- ^ Chorghade, Mukund S. (2006). Drug Discovery and Development, Volume 1: Drug Discovery. John Wiley & Sons. p. 201. ISBN 9780471780090.
- ^ Mosby’s Drug Consult: Lansoprazole
- ^ drugs.com International availability of lansoprazole Page accessed 3 February 2015
- ^ drugs.com Generic lansoprazole Page accessed 3 February 2015
- ^ “Prevacid Drug Profile”. Drugpatentwatch.com. Retrieved 30 April 2020.
- ^ Teva to release Prevacid version when patent expires
- ^ “Prevacid 24 HR- lansoprazole capsule, delayed release”. DailyMed. 7 August 2019. Retrieved 31 December 2019.
- ^ “Prevacid 24 HR- lansoprazole capsule, delayed release”. DailyMed. 11 December 2019. Retrieved 31 December 2019.
- ^ “Lansoprazole 24 HR- lansoprazole capsule, delayed release”. DailyMed. 21 December 2017. Retrieved 31 December 2019.
- ^ Jump up to:a b Villemagne, VL; Fodero-Tavoletti, MT; Masters, CL; Rowe, CC (January 2015). “Tau imaging: early progress and future directions”. The Lancet. Neurology. 14 (1): 114–24. doi:10.1016/s1474-4422(14)70252-2. PMID 25496902. S2CID 10502833.
External links
- “Lansoprazole”. Drug Information Portal. U.S. National Library of Medicine.
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /lænˈsoʊprəzoʊl/ lan-SOH-prə-zohl |
| Trade names | Prevacid, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695020 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth, intravenous (IV) |
| Drug class | Proton pump inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% or more |
| Protein binding | 97% |
| Metabolism | Liver (CYP3A4– and CYP2C19-mediated) |
| Elimination half-life | 1.0–1.5 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.173.220 |
| Chemical and physical data | |
| Formula | C16H14F3N3O2S |
| Molar mass | 369.36 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| (verify) | |