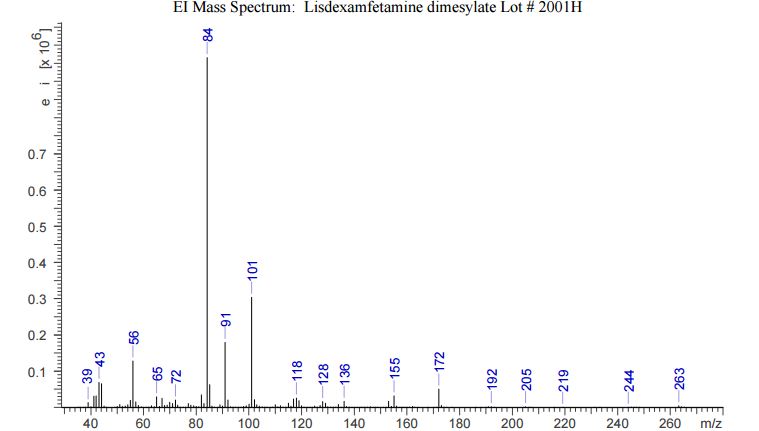
Lisdexamfetamine
- Molecular FormulaC15H25N3O
- Average mass263.379 Da

CAS 608137-33-3
(2S)-2,6-Diamino-N-[(1S)-1-methyl-2-phenylethyl]hexanamide dimethanesulfonate
https://www.google.com/patents/WO2005032474A2
| Applicants: | NEW RIVER PHARMACEUTICALS INC. [US/US]; The Governor Tyler, 1881 Grove Avenue, Radford, VA 24141 (US) (For All Designated States Except US). MICKLE, Travis [US/US]; (US) (For US Only). KRISHNAN, Suma [US/US]; (US) (For US Only). MONCRIEF, James, Scott [US/US]; (US) (For US Only). LAUDERBACK, Christopher [US/US]; (US) (For US Only). MILLER, Christal [US/US]; (US) (For US Only) |
| Inventors: | MICKLE, Travis; (US). KRISHNAN, Suma; (US). MONCRIEF, James, Scott; (US). LAUDERBACK, Christopher; (US). MILLER, Christal; (US) |

MICKLE, Travis
Dr. Travis Mickle founded KemPharm, Inc. in late 2006. Prior to KemPharm, from January 2003 to October 2005, Dr. Mickle was Director of Drug Discovery and Chemical Development at New River Pharmaceuticals where he also served in a variety of other senior research roles since joining the firm in 2001. During his tenure at New River, Dr. Mickle was responsible for creating a strong preclinical and clinical pipeline of drugs in the areas of ADHD, pain and thyroid dysfunction. His contributions included, as principal inventor, the discovery and development of lisdexamfetamine dimesylate, the highly successful therapy for the treatment of ADHD known as Vyvanse®. In addition, Dr. Mickle was an active participant in FDA and DEA meetings representing the company’s discovery/chemistry group and was also called in as a critical scientific resource during New River’s financings and strategic partnering discussions. Before his departure, Dr. Mickle played an integral part in New River’s development into a successful publicly-traded company which was subsequently acquired for $2.6 billion by its marketing partner, Shire PLC. Dr. Mickle is also the author of more than 150 US and international patents and patent applications, as well as several research papers. Dr. Mickle holds a Ph.D in Bio-Organic Chemistry from the University of Iowa.
ABOUT SUMA KRISHNAN
Mrs. Krishnan has served as our Senior Vice President — Regulatory Affairs since 2012. From 2009 to 2011, Mrs. Krishnan served as Senior Vice President of Product Development at Pinnacle Pharmaceuticals, Inc. From 2007 to 2009, she served as Chief Financial Officer of Light Matters Foundation. Previously, Mrs. Krishnan was Vice President, Product Development at New River Pharmaceuticals Inc. from September 2002 until its acquisition by Shire plc in April 2007.
Mrs. Krishnan has 22 years’ experience in drug development. Prior to serving at New River Pharmaceuticals Inc., Mrs. Krishnan served in the following capacities: Director, Regulatory Affairs at Shire Pharmaceuticals, Inc., a specialty pharmaceutical company; Senior Project Manager at Pfizer, Inc., a multi-national pharmaceutical company; and a consultant at the Weinberg Group, a pharmaceutical and environmental consulting firm.
Mrs. Krishnan began her career as a discovery scientist for Janssen Pharmaceuticals, Inc., a subsidiary of Johnson & Johnson, a multi-national pharmaceutical company, in May 1991. Mrs. Krishnan received an M.S. in Organic Chemistry from Villanova University, an M.B.A. from Institute of Management and Research (India) and an undergraduate degree in Organic Chemistry from Ferguson University (India).
Lisdexamfetamine (contracted from L–lysine–dextroamphetamine) is a prodrug of the central nervous system (CNS) stimulantdextroamphetamine, a phenethylamine of the amphetamine class that is used in the treatment of attention deficit hyperactivity disorder (ADHD) and binge eating disorder.[4][5] Its chemical structure consists of dextroamphetamine coupled with the essential amino acid L-lysine. Lisdexamfetamine itself is inactive prior to its absorption and the subsequent rate-limited enzymaticcleavage of the molecule’s L-lysine portion, which produces the active metabolite (dextroamphetamine).
Lisdexamfetamine can be prescribed for the treatment of attention deficit hyperactivity disorder (ADHD) in adults and children six and older, as well as for moderate to severe binge eating disorder in adults.[4] The safety and the efficacy of lisdexamfetamine dimesylate in children with ADHD three to five years old have not been established.[6]
Lisdexamfetamine is a Class B/Schedule II substance in the United Kingdom and a Schedule II controlled substance in the United States (DEA number 1205)[7] and the aggregate production quota for 2016 in the United States is 29,750 kilograms of anhydrous acid or base.[8] Lisdexamfetamine is currently in Phase III trials in Japan for ADHD.[9]
Uses
Medical
Lisdexamfetamine is used primarily as a treatment for attention deficit hyperactivity disorder (ADHD) and binge eating disorder;[4] it has similar off-label uses as those of other pharmaceutical amphetamines.[4][5] Long-term amphetamine exposure at sufficiently high doses in some animal species is known to produce abnormal dopamine system development or nerve damage,[10][11] but, in humans with ADHD, pharmaceutical amphetamines appear to improve brain development and nerve growth.[12][13][14] Reviews of magnetic resonance imaging (MRI) studies suggest that long-term treatment with amphetamine decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleus of the basal ganglia.[12][13][14]
Reviews of clinical stimulant research have established the safety and effectiveness of long-term continuous amphetamine use for the treatment of ADHD.[15][16][17] Randomized controlled trials of continuous stimulant therapy for the treatment of ADHD spanning two years have demonstrated treatment effectiveness and safety.[15][17] Two reviews have indicated that long-term continuous stimulant therapy for ADHD is effective for reducing the core symptoms of ADHD (i.e., hyperactivity, inattention, and impulsivity), enhancing quality of life and academic achievement, and producing improvements in a large number of functional outcomes[note 1] across nine outcome categories related to academics, antisocial behavior, driving, non-medicinal drug use, obesity, occupation, self-esteem, service use (i.e., academic, occupational, health, financial, and legal services), and social function.[16][17] One review highlighted a nine-month randomized controlled trial in children with ADHD that found an average increase of 4.5 IQ points, continued increases in attention, and continued decreases in disruptive behaviors and hyperactivity.[15] Another review indicated that, based upon the longest follow-up studies conducted to date, lifetime stimulant therapy that begins during childhood is continuously effective for controlling ADHD symptoms and reduces the risk of developing a substance use disorder as an adult.[17]
Current models of ADHD suggest that it is associated with functional impairments in some of the brain’s neurotransmitter systems;[18] these functional impairments involve impaired dopamine neurotransmission in the mesocorticolimbic projection and norepinephrine neurotransmission in the noradrenergic projections from the locus coeruleus to the prefrontal cortex.[18]Psychostimulants like methylphenidate and amphetamine are effective in treating ADHD because they increase neurotransmitter activity in these systems.[19][18][20] Approximately 80% of those who use these stimulants see improvements in ADHD symptoms.[21] Children with ADHD who use stimulant medications generally have better relationships with peers and family members, perform better in school, are less distractible and impulsive, and have longer attention spans.[22][23] The Cochrane Collaboration‘s reviews[note 2] on the treatment of ADHD in children, adolescents, and adults with pharmaceutical amphetamines stated that while these drugs improve short-term symptoms, they have higher discontinuation rates than non-stimulant medications due to their adverse side effects.[25][26] A Cochrane Collaboration review on the treatment of ADHD in children with tic disorders such as Tourette syndrome indicated that stimulants in general do not make tics worse, but high doses of dextroamphetamine could exacerbate tics in some individuals.[27]
Individuals over the age of 65 were not commonly tested in clinical trials of lisdexamfetamine for ADHD.[4] Lisdexamfetamine is being investigated for possible treatment of cognitive impairment associated with schizophrenia and excessive daytime sleepiness.[28]
Cognitive
In 2015, a systematic review and a meta-analysis of high quality clinical trials found that, when used at low (therapeutic) doses, amphetamine produces modest yet unambiguous improvements in cognition, including working memory, long-term episodic memory, inhibitory control, and some aspects of attention, in normal healthy adults;[29][30] these cognition-enhancing effects of amphetamine are known to be partially mediated through the indirect activation of both dopamine receptor D1 and adrenoceptor α2 in the prefrontal cortex.[19][29] A systematic review from 2014 found that low doses of amphetamine also improve memory consolidation, in turn leading to improved recall of information.[31] Therapeutic doses of amphetamine also enhance cortical network efficiency, an effect which mediates improvements in working memory in all individuals.[19][32]Amphetamine and other ADHD stimulants also improve task saliency (motivation to perform a task) and increase arousal (wakefulness), in turn promoting goal-directed behavior.[19][33][34] Stimulants such as amphetamine can improve performance on difficult and boring tasks and are used by some students as a study and test-taking aid.[19][34][35]Based upon studies of self-reported illicit stimulant use, 5–35% of college students use diverted ADHD stimulants, which are primarily used for performance enhancement rather than as recreational drugs.[36][37][38] However, high amphetamine doses that are above the therapeutic range can interfere with working memory and other aspects of cognitive control.[19][34]
Physical
Amphetamine is used by some athletes for its psychological and athletic performance-enhancing effects, such as increased endurance and alertness;[39][40] however, non-medical amphetamine use is prohibited at sporting events that are regulated by collegiate, national, and international anti-doping agencies.[41][42] In healthy people at oral therapeutic doses, amphetamine has been shown to increase muscle strength, acceleration, athletic performance in anaerobic conditions, and endurance (i.e., it delays the onset of fatigue), while improving reaction time.[39][43][44] Amphetamine improves endurance and reaction time primarily through reuptake inhibition and effluxion of dopamine in the central nervous system.[43][44][45] Amphetamine and other dopaminergic drugs also increase power output at fixed levels of perceived exertion by overriding a “safety switch” that allows the core temperature limit to increase in order to access a reserve capacity that is normally off-limits.[44][46][47] At therapeutic doses, the adverse effects of amphetamine do not impede athletic performance;[39][43] however, at much higher doses, amphetamine can induce effects that severely impair performance, such as rapid muscle breakdown and elevated body temperature.[48][49][43]
Available forms
Vyvanse capsules are available in doses of 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, and 70 mg of the active ingredient, lisdexamfetamine dimesylate.[50] Vyvanse capsules contain several inactive ingredients, including microcrystalline cellulose, croscarmellose sodium, and magnesium stearate.[50] The capsule shells contain gelatin and titanium dioxide, and may contain FD&C Red 3, FD&C Yellow 6, FD&C Blue 1, black iron oxide, and yellow iron oxide.[50]
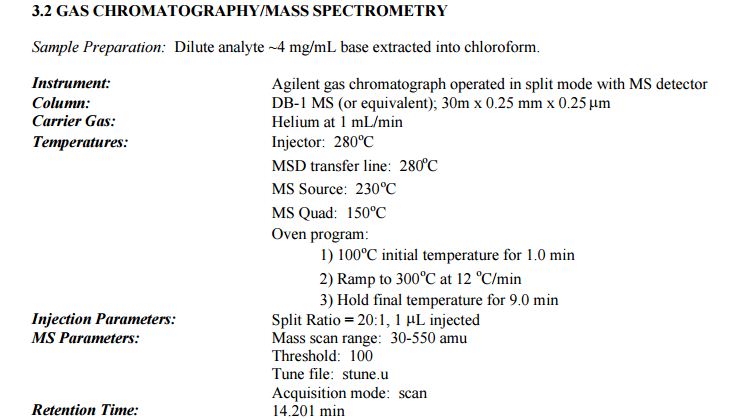
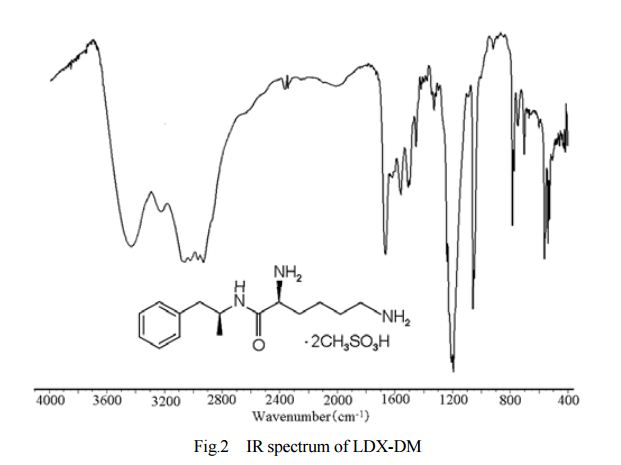
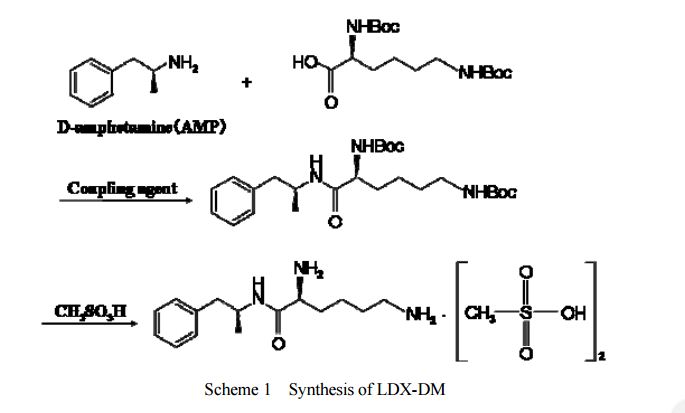
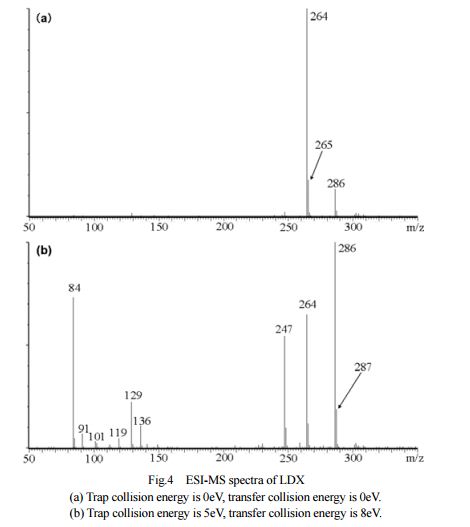
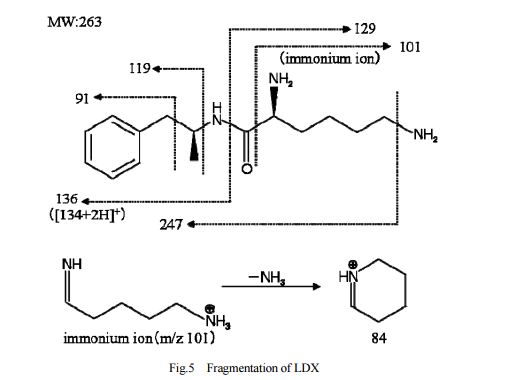
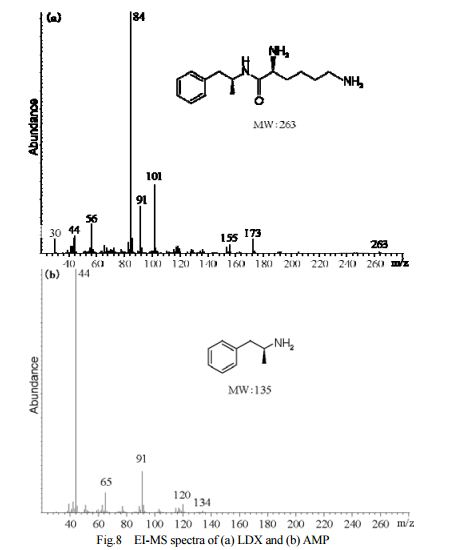
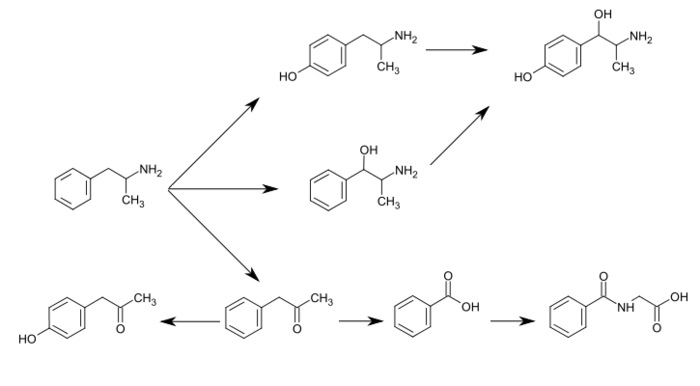
DESCRIPTION/SYNTHESIS
Lisdexamfetamine dimesylate is approved and marketed in the United States for the treatment of attention-deficit hyperactivity disorder in pediatric patients. The active compound lisdexamfetamine contains D-amphetamine covalently linked to the essential amino acid L-lysine. Controlled release of D-amphetamine, a psychostimulant, occurs following administration of lisdexamfetamine to a patient. The controlled release has been reported to occur through hydrolysis of the amide bond linking D-amphetamine and L-lysine.
A procedure for making lisdexamfetamine hydrochloride is described in U.S. Pat. No. 7,223,735 to Mickle et al. (hereinafter Mickle). The procedure involves reacting D-amphetamine with (S)-2,5-dioxopyrrolidin-1-yl 2,6-bis(tert-butoxycarbonylamino)hexanoate to form a lysine-amphetamine intermediate bearing tert-butylcarbamate protecting groups. This intermediate is treated with hydrochloric acid to remove the tert-butylcarbamate protecting groups and provide lisdexamfetamine as its hydrochloride salt. However, this procedure suffers several drawbacks that are problematic when carrying out large scale reactions, such as manufacturing scale, to prepare lisdexamfetamine.
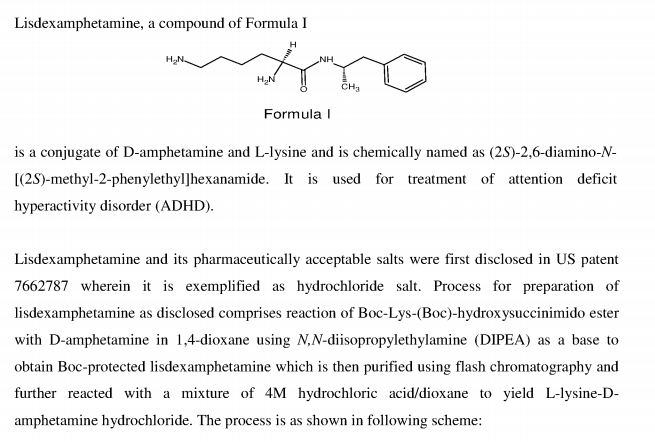
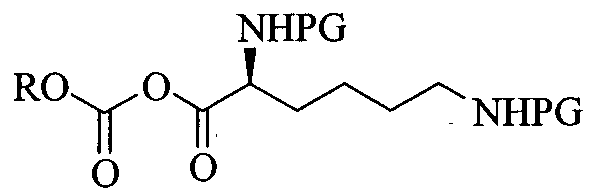
Lisdexamphetamine of formula I, is a conjugate of D-amphetamine and L-lysine and is chemically named as (2S)-2,6-diamino-N-[(lS)-methyl-2-phenylethyl]hexan amide.
Formula- I
Amphetamines stimulate central nervous system (CNS). Amphetamine is prescribed for treatment of various disorders, including attention deficit hyperactivity disorder (ADHD), obesity, nacrolepsy. It is approved as lisdextamphetamine dimesylate of formula IA and
Formula- IA
marketed under trade name Vyvanse for treatment of attention-deficit hyperactivity disorder in pediatric patients.
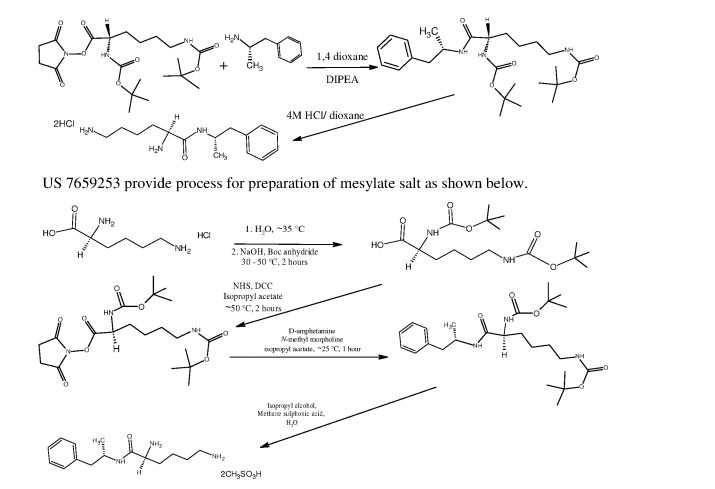
L-Lysine-D-amphetamine and its pharmaceutically acceptable salts were first disclosed in US patent 7,662,787 wherein it is exemplified as hydrochloride salt. Process for preparation of L- lysine-D-amphetamine includes reaction of BOC-Lys-(BOC)-hydroxysuccinimido ester with D-amphetamine in dioxane using diisopropyl ethyl amine (DIPEA) as a base to obtain BOC- protected lisdexamphetamine which is then purified using flash chromatography and further reacted with a mixture of 4M hydrochloric acid /dioxane to yield L-lysine-D-amphetamine hydrochloride. The process is as shown in following scheme:
4M HCI/dioxane
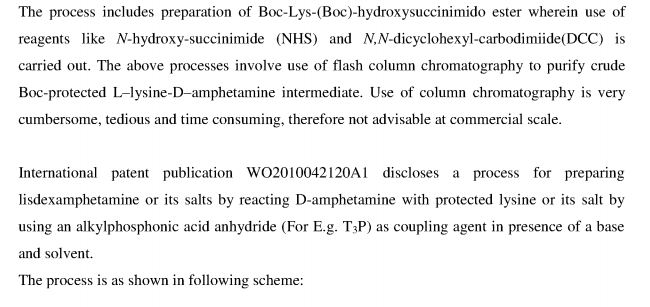
In equivalent patent US 7,659,253, process for preparation of mesylate salt is disclosed as shown below.
NHS;DCC;
isopropyl acetate
-50°C, 2 hr
Process includes preparation of BOC-Lys-(BOC)-hydroxysuccinimido ester wherein use of reagents like N-hydroxy-succinimide (NHS) and Ν,Ν-dicyclohexyl- carbodimiide (DCC) is carried out, The above processes involve use of flash column chromatography to purify crude BOC- protected L-lysine-D-amphetamine intermediate. Use of column chromatography is very cumbersome, tedoius and time consuming, therefore not advisable at commercial scale. Further Ν,Ν-dicyclohexylcarbodimiide is known to be highly toxic and moisture sensitive compound, and its use leads to formation of a large amount of Ν,Ν-dicyclohexyl urea (DCU) as bye product which has to be removed from reaction mixture.. Therefore use of DCC is not advisable at industrial scale.
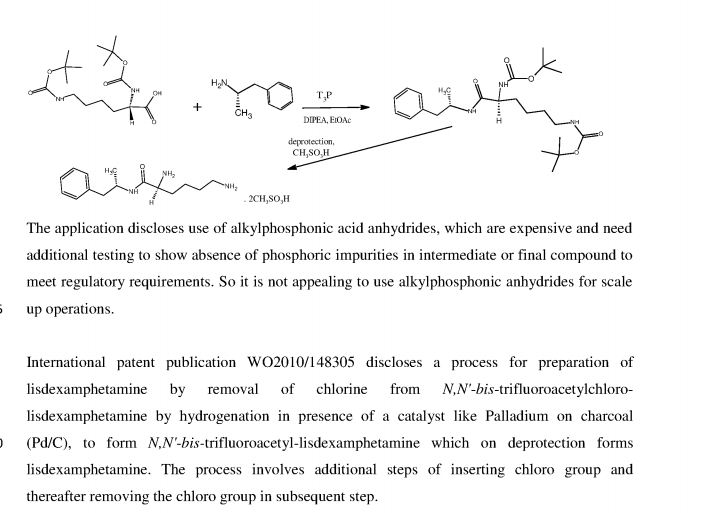
International patent publication WO 2010/042120 discloses a process for preparing L-lysine- D-amphetamine or its salts by reacting D-amphetamine with protected lysine or its salt by using an alkylphosphonic acid anhydride as coupling agent in presence of a base and solvent. The process is as shown in following scheme:
The application discloses use of alkylphosphonic acid anhydrides, which are expensive, and needs additional testing to show absence of phosphic impurities in intermediate or final compound to meet regulatory requirements. So it is not appealing to use alkylphosphonic anhydrides for scale up operations.
International patent publication WO2010/148305 discloses a process for preparation of lisdexamphetamine by removal of chlorine from Ν,Ν’-bistrifluoroacetyl-chloro- lisdexamphetamine by using hydrogenation catalyst like Pd/C, under hydrogen gas to form Ν,Ν’-bistrifluoroacetyl-lisdexamphetamine which on further deprotection by using deprotecting agent to form lisdexamphetamine. Alternatively first deprotection by using deprotecting agent and then chlorine is removed by using hydrogenation catalyst like Pd/C under hydrogen gas. The process involves additional steps of inserting chloro group and thereafter removing chloro group; further Pd/C is an expensive reagent, hence not attractive option from cost point of view.
It is therefore, necessary to overcome problems associated with prior art and to provide an efficient process for preparation of lisdexamphetamine and its pharmaceutically acceptable salts using easily available, less expensive, easy to handle raw materials and avoid use of column chromatography.
PATENT
https://www.google.com/patents/US20120157706


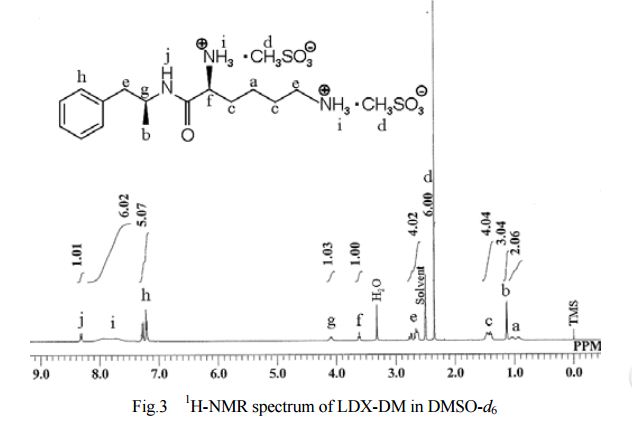
- Example IPreparation of N,N′-Biscarbobenzyloxy-Lisdexamfetamine (LDX-(Cbz)2)
General Experimental Procedure: In an appropriately sized, inert jacketed reactor charge 378.3 g of Cbz-Lys(Cbz)-OSu and 2309 g of isopropyl acetate. Heat the stirred slurry to ˜50° C. In a second reactor mix 100.0 g of D-amphetamine into 165 g of isopropyl acetate. Add the D-amphetamine solution to the batch over 1.75-2.25 hours. After the addition is complete stir the heterogeneous mixture at 50-55° C. until the reaction is complete by HPLC analysis. Charge 1197 g of methanol and heat the batch at a vigorous reflux 1 hour. Cool the batch to 45-55° C. over 2 hours then hold at temperature for 14-16 hours. Cool the batch to 15-25° C. at a rate of 5-10° C. per hour. Filter the slurry. Rinse the wet cake with 449 g of methanol and dry on the filter under nitrogen. To a clean, dry reactor charge the crude solids and 1642 g of methanol. Stir the slurry and heat to a vigorous reflux for 2 hours. Cool the batch to 45-55° C. over 1-2 hours then hold at temperature 12-16 hours. Continue cooling to 15-25° C. at a rate of 5-10° C. per hour. Filter the slurry and wash the wet cake with 547 g of methanol. Vacuum dry the wet cake at ˜55° C. to give product as a white to off-white solid (332 g, 88 mol %).
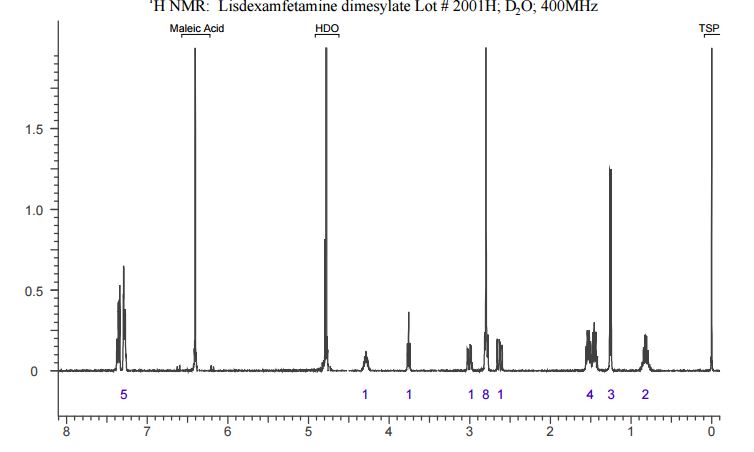
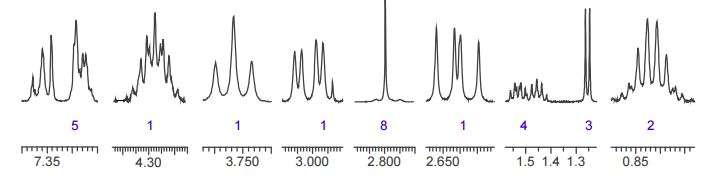
The crude LDX-(Cbz)product isolated from the reaction mixture by crystallization had a purity of 99.96% according to HPLC analysis.1H NMR analysis of crude LDX-(Cbz)2product revealed the presence of 0.57% by weight N-hydroxysuccinimide. The purified LDX-(Cbz)2product obtained by re-crystallization from methanol had a purity of 99.99% according to HPLC analysis.1H NMR analysis of the purified LDX-(Cbz)2product obtained by re-crystallization from methanol revealed that the amount of N-hydroxysuccinimide in the purified LDX-(Cbz)2product was reduced to 0.05% by weight.
Example 2Preparation of Lisdexamfetamine Dimesylate
General Experimental Procedure: In an appropriately sized, inert autoclave charge 100.0 g of LDX-(Cbz)2, 1 g of 10% (50% wet) palladium on carbon and 607.5 g of n-butanol. Stir the mixture under 100-150 psi of hydrogen at 80-85° C. until the reaction is complete by HPLC analysis. Heat the batch to 95-97° C. and hot filter. Transfer the product rich filtrate to an appropriately sized glass reactor. Charge 7.6 g of methanesulfonic acid maintaining a batch temperature of 32-38° C. To the resulting solution add 1.3 g of LDX-2MSA seed crystal. Stir the batch 4-16 hours at 32-38° C. Charge 30.4 g of methanesulfonic acid to the slurry over not less than 2 hours maintaining a batch temperature of 32-38° C. After the addition stir 1-2 hours at 32-38° C. Charge 436 g of isopropyl acetate over not less than 2 hours, then stir 1-2 hours at 32-38° C. Cool the batch to 15-25° C. and hold 1 hour. Filter the slurry. Wash the wet cake with a premixed combination of n-butanol (91.5 g) and isopropyl acetate (32.7 g) followed by a wash of isopropyl acetate (87.2 g). Vacuum dry the wet cake at ˜50° C. to give product as a white to off-white solid (78.8 g, 92 mol %).
PATENT
Sun Pharmaceutical Industries Ltd
Process for preparation of lisdexamphetamine and its salts via a novel aziridine intermediate is claimed. Lisdexamphetamine attention deficit hyperactivity disorder (ADHD). Represents the first patenting to be seen from Sun pharmaceutical that focuses on lisdexamphetamine. At the time of publication Pawar and Patel are affiliated with Chattem chemicals .
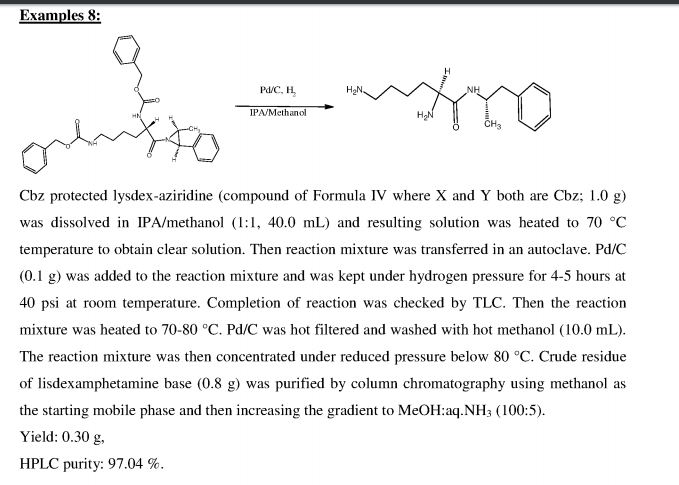
PATENT
IN 2011DE02040
WO 2013011526
IN 2009CH01986
WO 2010042120
https://www.google.com/patents/WO2013011526A1?cl=en

Formula II A
Formula IV
Formula IVA
EXAMPLES
Examplel Preparation of 2,6-bis-tertiarvbutoxycarbonylamino hexanoic acid
To a solution of L-lysine monohydrochloride (25g, 0.14mol) and sodium hydroxide (15g) in water (250 ml), ditertiary butyl dicarbonate (70.0 g, 0.32 mol) was added at 15-25°C. The temperature was slowly raised to 55-60 °C and the reaction mixture was stirred for 12 hours.. After completion of reaction, ( monitored by TLC), the reaction mixture was cooled to 10-
15°C and pH was adjusted to 2.5-3.5 with 2N hydrochloric acid. The reaction mass’ was then extracted with dichloromethane (2 x 125 ml) and combined organic layer was successively washed with water (150 ml) and brine (150 ml). Dichloromethane layer was distilled under vacuum at 30-40 °C to obtain 29.7g of title compound as a viscous oily mass having purity 96.5% by HPLC.
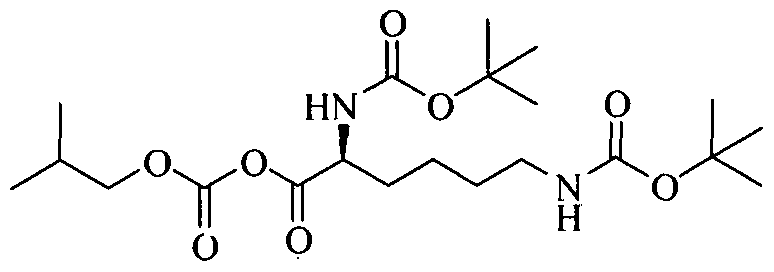
Example 2: Preparation of (5-tert-butoxycarbonylamino-5-(l-methyl-2-phenyl- ethylcarba moyl)-pentyll-carbamic acid tert-butyl ester;
To a solution of 2,6-bis-tertbutoxy carbonylamino hexanoic acid (7.5g) in dichloromethane (150 ml) , triethyl amine (8.0 ml) was added at 25-30°C and the reaction mixture was stirred for 15 minutes. The solution was cooled to -15 to -10°C and isobutylchloroformate (4.35 g) was slowly added under nitrogen atmosphere and stirred for 30 minutes at -15°C to – 10°C. A solution of D-amphetamine (3.85 g) in dichloromethane (10 ml) was slowly added and. the reaction mixture was stirred at 15 to -10°C for 60 minutes. The reaction completion was checked by TLC. After completion of the reaction temperature was raised to 25-30°C and reaction mixture was successively washed with 0.5 N hydrochloric acid solution (2 x 75 ml), sodium bicarbonate solution (5%w/w 75ml), water (50 ml) and brine solution (50 ml). The combined dichloromethane layer was dried over sodium sulfate (10.0 g) and distilled at 30- 40°C to obtain a semisolid compound which was stirred with a mixture of n-heptane (85 ml) and ethyl acetate (5ml) at 25-30°C for 30 minutes. The solid, thus obtained, was filtered and dried to get 10.21 g of title compound having purity 89.77% by HPLC. The crude compound was dissolved in ethanol (45ml) at 50-55°C and water (50ml) was added. The reaction mixture was slowly cooled to 35-40°C, stirred for 30 minutes. The solid, thus obtained, was filtered and dried to get 7.35g of pure title compound as a white crystalline solid having purity 99.5 % by HPLC.
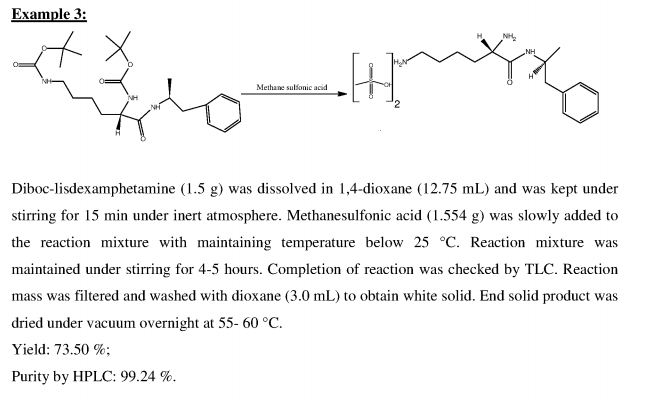
Example 3: Preparation of lisdexamphetamine dimesylate
(5-Tert-butoxycarbonylamino-5-( 1 -methyl-2-phenyl-ethylcarbamoyl)-pentyl]-carbamic acid tert-butyl ester (2.5g,) was dissolved in a mixture of isopropyl alcohol (10ml) and ethyl acetate (10ml) at 40-45°C and the reaction mass was cooled to 15-20°C. To this cold solution, methane sulphonic acid (2.5g) was added slowly and stirred for 12 hours at 15- 20°C. The reaction completion was checked by HPLC. The resulting solid was filtered, washed with a mixture of chilled isopropyl alcohol (5ml) and ethyl acetate (5ml) and dried under vacuum to obtain 1.68 g of title compound as a white crystalline solid having purity 99.72 % by HPLC.
Example 4: Preparation of lisdexamphetamine dimesylate:
(5-Tert-butoxycarbonylamino-5-( 1 -methyl-2-phenyl-ethylcarbamoyl)-pentyl]-carbamic acid tert-butyl ester (2.5 g,) was dissolved in ethanol (20 ml) at 25-30°C. To the reaction mixture, methane sulphonic acid (2.5 g) was slowly added and the reaction mixture was heated to 55- 60°C and stirred for 3 hours at 55-60°C. The reaction mixture was cooled to 25-30°C, stirred for 2 hours, filtered, washed with ethanol (10ml) and dried under vacuum to obtain 1.55g of lisdexamphetamine dimesylate having purity 99.61 % by HPLC.
Example 5: Purification of lisdexamphetamine dimesylate
Lisdexamphetamine dimesylate (1.40g,) was dissolved in ethanol (10 ml) at 50-55 °C and ethyl acetate (10 ml) was slowly added at 50-55 °C. The reaction mixture was cooled to 20- 25°C and stirred for 30 minutes. The resulting solid was filtered, washed with a mixture of ethanol and ethyl acetate (3 ml, 1: 1) and dried under vacuum at 55-60°C to obtain 1.28g of pure lisdexamphetamine dimesylate as a white crystalline solid having purity 99.90 % by HPLC.
Example 6: Preparation of lisdexamphetamine dimesylate
To a stirred solution of L-lysine monohydrochloride (50g) and sodium hydroxide (30 g) in water (500ml) at 15-25°C, ditertbutyl dicarbonate(140g) was added. The temperature was slowly raised to 55-60°C and reaction mixture was stirred for 12 hours. After completion of reaction, the reaction mixture was cooled to 10-15°C and pH was adjusted to 2.5-3.5 with 2N hydrochloric acid. The reaction mixture was then extracted with dichloromethane (2 x 250 ml) and combined dichloromethane layer was successively washed with water (300 ml) and brine (300 ml). To the organic layer triethyl amine (58g) in dichloromethane (500 ml) was added. The solution was cooled to -15 to -20°C and isobutylchloroformate (42.5 g) was slowly added at -15 to -20°C and stirred for 1 hour. A solution of D-amphetamine (41.85 g) in dichloromethane (100 ml) was slowly added to reaction mixture at -15 to -20 °C and stirred. After completion of the reaction, the reaction mixture was successively washed with 0.5 N hydrochloric solution (2 x 450 ml), sodium bicarbonate solution (5%w/w, 450 ml), water (450 ml) and brine solution (450 ml). The organic layer was dried over sodium sulfate and distilled under vacuum at 30-40°C to afford a residue. To this residue, ethanol (480 ml) was added followed by slow addition of methane sulphonic acid (55 g) under nitrogen atmosphere. The reaction temperature was raised 55-60°C and after completion of reaction, the reaction mixture was cooled to 20-25°C and stirred for 2 hours. The resulting solid was filtered, washed with ethanol (50 ml) and suck dried for 30 minutes, further washed with ethanol and dried to obtain lisdexamphetamine dimesylate.
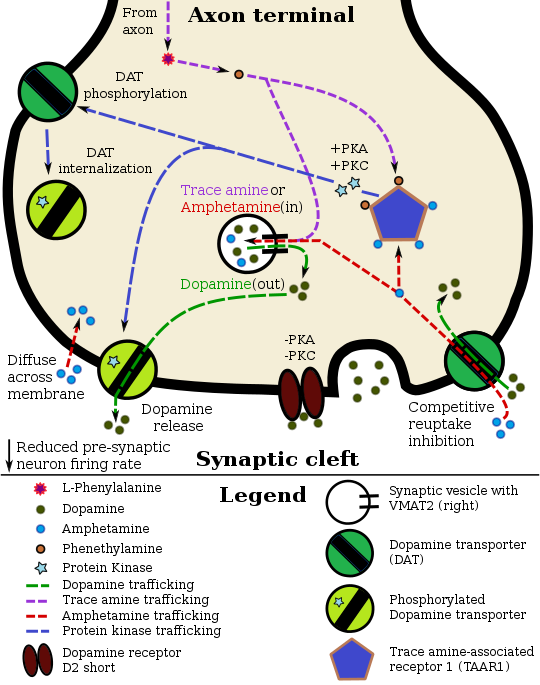
Mechanism of action
Amphetamine enters the presynaptic neuron across the neuronal membrane or through DAT. Once inside, it binds to TAAR1 or enters synaptic vesicles through VMAT2. When amphetamine enters synaptic vesicles through VMAT2, it collapses the vesicular pH gradient, which in turn causes dopamine to be released into the cytosol (light tan-colored area) through VMAT2. When amphetamine binds to TAAR1, it reduces the firing rate of the dopamine neuron via potassium channels and activates protein kinase A (PKA) and protein kinase C (PKC), which subsequently phosphorylate DAT. PKA-phosphorylation causes DAT to withdraw into the presynaptic neuron (internalize) and cease transport. PKC-phosphorylated DAT may either operate in reverse or, like PKA-phosphorylated DAT, internalize and cease transport. Amphetamine is also known to increase intracellular calcium, an effect which is associated with DAT phosphorylation through a CAMKIIα-dependent pathway, in turn producing dopamine efflux.
Lisdexamfetamine is an inactive prodrug that is converted in the body to dextroamphetamine, a pharmacologically active compound which is responsible for the drug’s activity.[119] After oral ingestion, lisdexamfetamine is broken down by enzymes in red blood cells to form L-lysine, a naturally occurring essential amino acid, and dextroamphetamine.[4] The conversion of lisdexamfetamine to dextroamphetamine is not affected by gastrointestinal pH and is unlikely to be affected by alterations in normal gastrointestinal transit times.[4][120]
The optical isomers of amphetamine, i.e., dextroamphetamine and levoamphetamine, are TAAR1 agonists and vesicular monoamine transporter 2 inhibitors that can enter monoamine neurons;[121][122] this allows them to release monoamine neurotransmitters (dopamine, norepinephrine, and serotonin, among others) from their storage sites in the presynaptic neuron, as well as prevent the reuptake of these neurotransmitters from the synaptic cleft.[121][122]
Lisdexamfetamine was developed with the goal of providing a long duration of effect that is consistent throughout the day, with reduced potential for abuse. The attachment of the amino acid lysine slows down the relative amount of dextroamphetamine available to the blood stream. Because no free dextroamphetamine is present in lisdexamfetamine capsules, dextroamphetamine does not become available through mechanical manipulation, such as crushing or simple extraction. A relatively sophisticated biochemical process is needed to produce dextroamphetamine from lisdexamfetamine.[120] As opposed to Adderall, which contains roughly equal parts of racemic amphetamine and dextroamphetamine salts, lisdexamfetamine is a single-enantiomer dextroamphetamine formula.[119][123] Studies conducted show that lisdexamfetamine dimesylate may have less abuse potential than dextroamphetamine and an abuse profile similar to diethylpropion at dosages that are FDA-approved for treatment of ADHD, but still has a high abuse potential when this dosage is exceeded by over 100%.[120]
Pharmacokinetics
The oral bioavailability of amphetamine varies with gastrointestinal pH;[118] it is well absorbed from the gut, and bioavailability is typically over 75% for dextroamphetamine.[124] Amphetamine is a weak base with a pKa of 9.9;[125]consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium.[125][118] Conversely, an acidic pH means the drug is predominantly in a water-soluble cationic (salt) form, and less is absorbed.[125] Approximately 15–40% of amphetamine circulating in the bloodstream is bound to plasma proteins.[126]
The half-life of amphetamine enantiomers differ and vary with urine pH.[125] At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively.[125] An acidic diet will reduce the enantiomer half-lives to 8–11 hours; an alkaline diet will increase the range to 16–31 hours.[127][128] The biological half-life is longer and distribution volumes are larger in amphetamine dependent individuals.[128] The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively.[125] Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH.[125] When the urinary pH is basic, amphetamine is in its free base form, so less is excreted.[125] When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively.[125] Amphetamine is usually eliminated within two days of the last oral dose.[127]
The prodrug lisdexamfetamine is not as sensitive to pH as amphetamine when being absorbed in the gastrointestinal tract;[129] following absorption into the blood stream, it is converted by red blood cell-associated enzymes to dextroamphetamine via hydrolysis.[129] The elimination half-life of lisdexamfetamine is generally less than one hour.[129]
CYP2D6, dopamine β-hydroxylase (DBH), flavin-containing monooxygenase 3 (FMO3), butyrate-CoA ligase (XM-ligase), and glycine N-acyltransferase (GLYAT) are the enzymes known to metabolize amphetamine or its metabolites in humans.[sources 9] Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone.[125][127][134] Among these metabolites, the active sympathomimetics are 4‑hydroxyamphetamine,[138] 4‑hydroxynorephedrine,[139] and norephedrine.[140] The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination.[125][127] The known metabolic pathways, detectable metabolites, and metabolizing enzymes in humans include the following:
Hydroxylation
Hydroxylation
Hydroxylation
Hydroxylation
Hydroxylation
Deamination
Conjugation
The primary active metabolites of amphetamine are 4-hydroxyamphetamine and norephedrine;[134] at normal urine pH, about 30–40% of amphetamine is excreted unchanged and roughly 50% is excreted as the inactive metabolites (bottom row).[125] The remaining 10–20% is excreted as the active metabolites.[125] Benzoic acid is metabolized by XM-ligase into an intermediate product, benzoyl-CoA,[136] which is then metabolized by GLYAT into hippuric acid.[137]
Chemistry
Comparison to other formulationsLisdexamfetamine dimesylate is a water-soluble (792 mg/mL) powder with a white to off-white color.[50]
Lisdexamfetamine dimesylate is one marketed formulation delivering dextroamphetamine. The following table compares the drug to other amphetamine pharmaceuticals.
| drug | formula | molecular mass [note 8] |
amphetamine base [note 9] |
amphetamine base in equal doses |
doses with equal base content [note 10] |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[142][143] | (C9H13N)2•H2SO4 | |||||||||
| amphetamine sulfate[144] | (C9H13N)2•H2SO4 | |||||||||
| Adderall | ||||||||||
| 25% | dextroamphetamine sulfate[142][143] | (C9H13N)2•H2SO4 | ||||||||
| 25% | amphetamine sulfate[144] | (C9H13N)2•H2SO4 | ||||||||
| 25% | dextroamphetamine saccharate[145] | (C9H13N)2•C6H10O8 | ||||||||
| 25% | amphetamine aspartate monohydrate[146] | (C9H13N)•C4H7NO4•H2O | ||||||||
| lisdexamfetamine dimesylate[147] | C15H25N3O•(CH4O3S)2 | |||||||||
| amphetamine base suspension[note 11][56] | C9H13N | |||||||||
History, society, and culture
Lisdexamfetamine was developed by New River Pharmaceuticals, who were bought by Shire Pharmaceuticals shortly before lisdexamfetamine began being marketed. It was developed for the intention of creating a longer-lasting and less-easily abused version of dextroamphetamine, as the requirement of conversion into dextroamphetamine via enzymes in the red blood cells increases its duration of action, regardless of the route of ingestion.[148] The drug lisdexamfetamine dimesylate is the first prodrug of its kind.
On 23 April 2008, Vyvanse received FDA approval for the adult population.[149] On 19 February 2009, Health Canada approved 30 mg and 50 mg capsules of lisdexamfetamine for treatment of ADHD.[150] On 8 February 2012, Vyvanse received FDA approval for maintenance treatment of adult ADHD.[151] In February 2014, Shire announced that two late-stage clinical trials had shown that Vyvanse was not an effective treatment for depression.[152] Lisdexamfetamine was granted approval in a number of European countries for the treatment of ADHD in children and adolescents over the age of 6 years, as well as adults who are continuing treatment from childhood, after a positive outcome of the regulatory procedure.[153] Shire also recently announced receipt of a positive result from a European decentralised procedure for lisdexamfetamine for adult patients with ADHD in the United Kingdom, Sweden and Denmark, expanding the indication of lisdexamfetamine to include newly diagnosed adult patients.[154]
In January 2015, lisdexamfetamine was approved by the U.S. Food and Drug Administration for treatment of binge eating disorder in adults.[28][155][156]
In January 2017, a new dosage form of lisdexamfetamine in the form of a chewable tablet (as opposed to a capsule) was approved by the FDA.[157]
Brand names
Lisdexamfetamine is sold as Tyvense (IE), Elvanse (UK), Venvanse (BR), Vyvanse (CA, US).[158]
Clinical research
Some clinical trials that used lisdexamfetamine as an add-on therapy with a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) for treatment-resistant depression indicated that this is no more effective than the use of an SSRI or SNRI alone.[159] Other studies indicated that psychostimulants potentiated antidepressants, and were under-prescribed for treatment resistant depression. In those studies patients showed significant improvement in energy, mood, and psychomotor activity.[160]
Notes
- Jump up^ The ADHD-related outcome domains with the greatest proportion of significantly improved outcomes from long-term continuous stimulant therapy include academics (~55% of academic outcomes improved), driving (100% of driving outcomes improved), non-medical drug use (47% of addiction-related outcomes improved), obesity (~65% of obesity-related outcomes improved), self esteem (50% of self-esteem outcomes improved), and social function (67% of social function outcomes improved).[16]The largest effect sizes for outcome improvements from long-term stimulant therapy occur in the domains involving academics (e.g., grade point average, achievement test scores, length of education, and education level), self-esteem (e.g., self-esteem questionnaire assessments, number of suicide attempts, and suicide rates), and social function (e.g., peer nomination scores, social skills, and quality of peer, family, and romantic relationships).[16]Long-term combination therapy for ADHD (i.e., treatment with both a stimulant and behavioral therapy) produces even larger effect sizes for outcome improvements and improves a larger proportion of outcomes across each domain compared to long-term stimulant therapy alone.[16]
- Jump up^ Cochrane Collaboration reviews are high quality meta-analytic systematic reviews of randomized controlled trials.[24]
- Jump up^ The 95% confidence interval indicates that there is a 95% probability that the true number of deaths lies between 3,425 and 4,145.
- Jump up^ Transcription factors are proteins that increase or decrease the expression of specific genes.[93]
- Jump up^ In simpler terms, this necessary and sufficient relationship means that ΔFosB overexpression in the nucleus accumbens and addiction-related behavioral and neural adaptations always occur together and never occur alone.
- Jump up^ NMDA receptors are voltage-dependent ligand-gated ion channels that requires simultaneous binding of glutamate and a co-agonist (d-serine or glycine) to open the ion channel.[105]
- Jump up^ The review indicated that magnesium L-aspartate and magnesium chloride produce significant changes in addictive behavior;[71] other forms of magnesium were not mentioned.
- Jump up^ For uniformity, molecular masses were calculated using the Lenntech Molecular Weight Calculator[141] and were within 0.01g/mol of published pharmaceutical values.
- Jump up^ Amphetamine base percentage = molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- Jump up^ dose = (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- Jump up^ This product (Dyanavel XR) is an oral suspension (i.e., a drug that is suspended in a liquid and taken by mouth) that contains 2.5 mg/mL of amphetamine base.[56] The amphetamine base contains dextro- to levo-amphetamine in a ratio of 3.2:1,[56] which is approximately the ratio in Adderall. The product uses an ion exchange resin to achieve extended release of the amphetamine base.[56]
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US20050038121 * | Jun 1, 2004 | Feb 17, 2005 | New River Pharmaceuticals Inc. | Abuse resistant lysine amphetamine compounds |
| US20110196173 * | Oct 9, 2008 | Aug 11, 2011 | Andreas Meudt | Process for the Synthesis of Amphetamine Derivatives |
| DE1493824A1 * | Nov 23, 1964 | May 22, 1969 | Hoffmann La Roche | Verfahren zur Herstellung von Aminocarbonsaeureamiden |
| Reference | ||
|---|---|---|
| 1 | * | “Benzyl Chloroformate” in Handbook of Reagents for Organic Synthesis – Activating Agents and Protecting Groups ; Pearson et al., eds., 1999 John Wiley & Sons, pp. 46-50 |
| 2 | * | DATABASE CAPLUS CHEMICAL ABSTRACTS SERVICE, COLUMBUS, OHIO, US; Database Accession No. 1966:19805, Abstract NL 6414901, 28 July 1965 |
| 3 | * | Smith and March. Advanced Organic Chemistry 6th ed. (501-502) |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US8614346 | Jun 18, 2010 | Dec 24, 2013 | Cambrex Charles City, Inc. | Methods and compositions for preparation of amphetamine conjugates and salts thereof |
| WO2017003721A1 | Jun 17, 2016 | Jan 5, 2017 | Noramco, Inc. | Process for the preparation of lisdexamfetamine and related derivatives |
References
- Jump up^ “Public Assessment Report Decentralised Procedure” (PDF). Shire Pharmaceuticals Contracts Limited. p. 14. Retrieved 23 August 2014.
- ^ Jump up to:a b Millichap JG (2010). “Chapter 9: Medications for ADHD”. In Millichap JG. Attention Deficit Hyperactivity Disorder Handbook: A Physician’s Guide to ADHD (2nd ed.). New York, USA: Springer. p. 112. ISBN 9781441913968.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] …
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] …
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - ^ Jump up to:a b Brams M, Mao AR, Doyle RL (September 2008). “Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder”. Postgrad. Med. 120 (3): 69–88. PMID 18824827. doi:10.3810/pgm.2008.09.1909.
Onset of efficacy was earliest for d-MPH-ER at 0.5 hours, followed by d, l-MPH-LA at 1 to 2 hours, MCD at 1.5 hours, d, l-MPH-OR at 1 to 2 hours, MAS-XR at 1.5 to 2 hours, MTS at 2 hours, and LDX at approximately 2 hours. … MAS-XR, and LDX have a long duration of action at 12 hours postdose
- ^ Jump up to:a b c d e f g h i j k l m “Vyvanse Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. January 2015. Retrieved 24 February 2015.
- ^ Jump up to:a b Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). “Amphetamine, past and present – a pharmacological and clinical perspective”. J. Psychopharmacol. 27 (6): 479–496. PMC 3666194
 . PMID 23539642. doi:10.1177/0269881113482532.
. PMID 23539642. doi:10.1177/0269881113482532. - Jump up^ “Lisdexamfetamine dimesylate (generic).” Brown University Psychopharmacology Update 19.7 (2008): 1–2. Academic Search Premier. EBSCO. Web. 12 September 2010.
- Jump up^ “DEA – Department of Justice” (PDF). DEA – Department of Justice. Retrieved 1 July 2014.
- Jump up^ “DEA Office of Diversion Control” (PDF). DEA. Retrieved 1 July 2014.
- Jump up^ “Phase-III clinical trials in Attention-deficit hyperactivity disorder (In children, In adolescents) in Japan (PO)”. Retrieved 20 March 2016.
- ^ Jump up to:a b Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, Bastos Mde L (August 2012). “Toxicity of amphetamines: an update”. Arch. Toxicol. 86 (8): 1167–1231. PMID 22392347. doi:10.1007/s00204-012-0815-5.
- Jump up^ Berman S, O’Neill J, Fears S, Bartzokis G, London ED (October 2008). “Abuse of amphetamines and structural abnormalities in the brain”. Ann. N. Y. Acad. Sci. 1141: 195–220. PMC 2769923
 . PMID 18991959. doi:10.1196/annals.1441.031.
. PMID 18991959. doi:10.1196/annals.1441.031. - ^ Jump up to:a b Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). “Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects”. JAMA Psychiatry. 70 (2): 185–198. PMID 23247506. doi:10.1001/jamapsychiatry.2013.277.
- ^ Jump up to:a b Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J (September 2013). “Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies”. J. Clin. Psychiatry. 74 (9): 902–917. PMC 3801446
 . PMID 24107764. doi:10.4088/JCP.12r08287.
. PMID 24107764. doi:10.4088/JCP.12r08287. - ^ Jump up to:a b Frodl T, Skokauskas N (February 2012). “Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects.”. Acta psychiatrica Scand. 125 (2): 114–126. PMID 22118249. doi:10.1111/j.1600-0447.2011.01786.x.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- ^ Jump up to:a b c Millichap JG (2010). “Chapter 9: Medications for ADHD”. In Millichap JG. Attention Deficit Hyperactivity Disorder Handbook: A Physician’s Guide to ADHD (2nd ed.). New York, USA: Springer. pp. 121–123, 125–127. ISBN 9781441913968.
Ongoing research has provided answers to many of the parents’ concerns, and has confirmed the effectiveness and safety of the long-term use of medication.
- ^ Jump up to:a b c d e Arnold LE, Hodgkins P, Caci H, Kahle J, Young S (February 2015). “Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review”. PLoS ONE. 10 (2): e0116407. PMC 4340791
 . PMID 25714373. doi:10.1371/journal.pone.0116407.
. PMID 25714373. doi:10.1371/journal.pone.0116407. The highest proportion of improved outcomes was reported with combination treatment (83% of outcomes). Among significantly improved outcomes, the largest effect sizes were found for combination treatment. The greatest improvements were associated with academic, self-esteem, or social function outcomes.
Figure 3: Treatment benefit by treatment type and outcome group - ^ Jump up to:a b c d Huang YS, Tsai MH (July 2011). “Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge”. CNS Drugs. 25 (7): 539–554. PMID 21699268. doi:10.2165/11589380-000000000-00000.
Recent studies have demonstrated that stimulants, along with the non-stimulants atomoxetine and extended-release guanfacine, are continuously effective for more than 2-year treatment periods with few and tolerable adverse effects. The effectiveness of long-term therapy includes not only the core symptoms of ADHD, but also improved quality of life and academic achievements. The most concerning short-term adverse effects of stimulants, such as elevated blood pressure and heart rate, waned in long-term follow-up studies. … In the longest follow-up study (of more than 10 years), lifetime stimulant treatment for ADHD was effective and protective against the development of adverse psychiatric disorders.
- ^ Jump up to:a b c Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 154–157. ISBN 9780071481274.
- ^ Jump up to:a b c d e f Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 13: Higher Cognitive Function and Behavioral Control”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 318, 321. ISBN 9780071481274.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in normal subjects and those with ADHD. … stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks … through indirect stimulation of dopamine and norepinephrine receptors. …
Beyond these general permissive effects, dopamine (acting via D1 receptors) and norepinephrine (acting at several receptors) can, at optimal levels, enhance working memory and aspects of attention. - Jump up^ Bidwell LC, McClernon FJ, Kollins SH (August 2011). “Cognitive enhancers for the treatment of ADHD”. Pharmacol. Biochem. Behav. 99 (2): 262–274. PMC 3353150
 . PMID 21596055. doi:10.1016/j.pbb.2011.05.002.
. PMID 21596055. doi:10.1016/j.pbb.2011.05.002. - Jump up^ Parker J, Wales G, Chalhoub N, Harpin V (September 2013). “The long-term outcomes of interventions for the management of attention-deficit hyperactivity disorder in children and adolescents: a systematic review of randomized controlled trials”. Psychol. Res. Behav. Manag. 6: 87–99. PMC 3785407
 . PMID 24082796. doi:10.2147/PRBM.S49114.
. PMID 24082796. doi:10.2147/PRBM.S49114. Only one paper53 examining outcomes beyond 36 months met the review criteria. … There is high level evidence suggesting that pharmacological treatment can have a major beneficial effect on the core symptoms of ADHD (hyperactivity, inattention, and impulsivity) in approximately 80% of cases compared with placebo controls, in the short term.
- Jump up^ Millichap JG (2010). “Chapter 9: Medications for ADHD”. In Millichap JG. Attention Deficit Hyperactivity Disorder Handbook: A Physician’s Guide to ADHD (2nd ed.). New York, USA: Springer. pp. 111–113. ISBN 9781441913968.
- Jump up^ “Stimulants for Attention Deficit Hyperactivity Disorder”. WebMD. Healthwise. 12 April 2010. Retrieved 12 November 2013.
- Jump up^ Scholten RJ, Clarke M, Hetherington J (August 2005). “The Cochrane Collaboration”. Eur. J. Clin. Nutr. 59 Suppl 1: S147–S149; discussion S195–S196. PMID 16052183. doi:10.1038/sj.ejcn.1602188.
- ^ Jump up to:a b Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M (June 2011). Castells X, ed. “Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults”. Cochrane Database Syst. Rev. (6): CD007813. PMID 21678370. doi:10.1002/14651858.CD007813.pub2.
- Jump up^ Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, Vohra S (February 2016). “Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents”. Cochrane Database Syst. Rev. 2: CD009996. PMID 26844979. doi:10.1002/14651858.CD009996.pub2.
- Jump up^ Pringsheim T, Steeves T (April 2011). Pringsheim T, ed. “Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders”. Cochrane Database Syst. Rev. (4): CD007990. PMID 21491404. doi:10.1002/14651858.CD007990.pub2.
- ^ Jump up to:a b http://www.shire.com/shireplc/en/rd/pipeline
- ^ Jump up to:a b Spencer RC, Devilbiss DM, Berridge CW (June 2015). “The Cognition-Enhancing Effects of Psychostimulants Involve Direct Action in the Prefrontal Cortex”. Biol. Psychiatry. 77 (11): 940–950. PMC 4377121
 . PMID 25499957. doi:10.1016/j.biopsych.2014.09.013.
. PMID 25499957. doi:10.1016/j.biopsych.2014.09.013. The procognitive actions of psychostimulants are only associated with low doses. Surprisingly, despite nearly 80 years of clinical use, the neurobiology of the procognitive actions of psychostimulants has only recently been systematically investigated. Findings from this research unambiguously demonstrate that the cognition-enhancing effects of psychostimulants involve the preferential elevation of catecholamines in the PFC and the subsequent activation of norepinephrine α2 and dopamine D1 receptors. … This differential modulation of PFC-dependent processes across dose appears to be associated with the differential involvement of noradrenergic α2 versus α1 receptors. Collectively, this evidence indicates that at low, clinically relevant doses, psychostimulants are devoid of the behavioral and neurochemical actions that define this class of drugs and instead act largely as cognitive enhancers (improving PFC-dependent function). … In particular, in both animals and humans, lower doses maximally improve performance in tests of working memory and response inhibition, whereas maximal suppression of overt behavior and facilitation of attentional processes occurs at higher doses.
- Jump up^ Ilieva IP, Hook CJ, Farah MJ (January 2015). “Prescription Stimulants’ Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis”. J. Cogn. Neurosci. 27: 1–21. PMID 25591060. doi:10.1162/jocn_a_00776.
Specifically, in a set of experiments limited to high-quality designs, we found significant enhancement of several cognitive abilities. … The results of this meta-analysis … do confirm the reality of cognitive enhancing effects for normal healthy adults in general, while also indicating that these effects are modest in size.
- Jump up^ Bagot KS, Kaminer Y (April 2014). “Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review”. Addiction. 109 (4): 547–557. PMC 4471173
 . PMID 24749160. doi:10.1111/add.12460.
. PMID 24749160. doi:10.1111/add.12460. Amphetamine has been shown to improve consolidation of information (0.02 ≥ P ≤ 0.05), leading to improved recall.
- Jump up^ Devous MD, Trivedi MH, Rush AJ (April 2001). “Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers”. J. Nucl. Med. 42 (4): 535–542. PMID 11337538.
- Jump up^ Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 266. ISBN 9780071481274.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ^ Jump up to:a b c Wood S, Sage JR, Shuman T, Anagnostaras SG (January 2014). “Psychostimulants and cognition: a continuum of behavioral and cognitive activation”. Pharmacol. Rev. 66 (1): 193–221. PMID 24344115. doi:10.1124/pr.112.007054.
- Jump up^ Twohey M (26 March 2006). “Pills become an addictive study aid”. JS Online. Archived from the original on 15 August 2007. Retrieved 2 December 2007.
- Jump up^ Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ (October 2006). “Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration”. Pharmacotherapy. 26 (10): 1501–1510. PMC 1794223
 . PMID 16999660. doi:10.1592/phco.26.10.1501.
. PMID 16999660. doi:10.1592/phco.26.10.1501. - Jump up^ Weyandt LL, Oster DR, Marraccini ME, Gudmundsdottir BG, Munro BA, Zavras BM, Kuhar B (September 2014). “Pharmacological interventions for adolescents and adults with ADHD: stimulant and nonstimulant medications and misuse of prescription stimulants”. Psychol. Res. Behav. Manag. 7: 223–249. PMC 4164338
 . PMID 25228824. doi:10.2147/PRBM.S47013.
. PMID 25228824. doi:10.2147/PRBM.S47013. misuse of prescription stimulants has become a serious problem on college campuses across the US and has been recently documented in other countries as well. … Indeed, large numbers of students claim to have engaged in the nonmedical use of prescription stimulants, which is reflected in lifetime prevalence rates of prescription stimulant misuse ranging from 5% to nearly 34% of students.
- Jump up^ Clemow DB, Walker DJ (September 2014). “The potential for misuse and abuse of medications in ADHD: a review”. Postgrad. Med. 126 (5): 64–81. PMID 25295651. doi:10.3810/pgm.2014.09.2801.
Overall, the data suggest that ADHD medication misuse and diversion are common health care problems for stimulant medications, with the prevalence believed to be approximately 5% to 10% of high school students and 5% to 35% of college students, depending on the study.
- ^ Jump up to:a b c Liddle DG, Connor DJ (June 2013). “Nutritional supplements and ergogenic AIDS”. Prim. Care. 40 (2): 487–505. PMID 23668655. doi:10.1016/j.pop.2013.02.009.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training …
Physiologic and performance effects
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - ^ Jump up to:a b c d e f g h i j k l m n o p q r s Westfall DP, Westfall TC (2010). “Miscellaneous Sympathomimetic Agonists”. In Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s Pharmacological Basis of Therapeutics (12th ed.). New York, USA: McGraw-Hill. ISBN 9780071624428.
- Jump up^ Bracken NM (January 2012). “National Study of Substance Use Trends Among NCAA College Student-Athletes” (PDF). NCAA Publications. National Collegiate Athletic Association. Retrieved 8 October 2013.
- Jump up^ Docherty JR (June 2008). “Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)”. Br. J. Pharmacol. 154 (3): 606–622. PMC 2439527
 . PMID 18500382. doi:10.1038/bjp.2008.124.
. PMID 18500382. doi:10.1038/bjp.2008.124. - ^ Jump up to:a b c d Parr JW (July 2011). “Attention-deficit hyperactivity disorder and the athlete: new advances and understanding”. Clin. Sports Med. 30 (3): 591–610. PMID 21658550. doi:10.1016/j.csm.2011.03.007.
In 1980, Chandler and Blair47 showed significant increases in knee extension strength, acceleration, anaerobic capacity, time to exhaustion during exercise, pre-exercise and maximum heart rates, and time to exhaustion during maximal oxygen consumption (VO2 max) testing after administration of 15 mg of dextroamphetamine versus placebo. Most of the information to answer this question has been obtained in the past decade through studies of fatigue rather than an attempt to systematically investigate the effect of ADHD drugs on exercise.
- ^ Jump up to:a b c Roelands B, de Koning J, Foster C, Hettinga F, Meeusen R (May 2013). “Neurophysiological determinants of theoretical concepts and mechanisms involved in pacing”. Sports Med. 43 (5): 301–311. PMID 23456493. doi:10.1007/s40279-013-0030-4.
In high-ambient temperatures, dopaminergic manipulations clearly improve performance. The distribution of the power output reveals that after dopamine reuptake inhibition, subjects are able to maintain a higher power output compared with placebo. … Dopaminergic drugs appear to override a safety switch and allow athletes to use a reserve capacity that is ‘off-limits’ in a normal (placebo) situation.
- Jump up^ Parker KL, Lamichhane D, Caetano MS, Narayanan NS (October 2013). “Executive dysfunction in Parkinson’s disease and timing deficits”. Front. Integr. Neurosci. 7: 75. PMC 3813949
 . PMID 24198770. doi:10.3389/fnint.2013.00075.
. PMID 24198770. doi:10.3389/fnint.2013.00075. Manipulations of dopaminergic signaling profoundly influence interval timing, leading to the hypothesis that dopamine influences internal pacemaker, or “clock,” activity. For instance, amphetamine, which increases concentrations of dopamine at the synaptic cleft advances the start of responding during interval timing, whereas antagonists of D2 type dopamine receptors typically slow timing;… Depletion of dopamine in healthy volunteers impairs timing, while amphetamine releases synaptic dopamine and speeds up timing.
- Jump up^ Rattray B, Argus C, Martin K, Northey J, Driller M (March 2015). “Is it time to turn our attention toward central mechanisms for post-exertional recovery strategies and performance?”. Front. Physiol. 6: 79. PMC 4362407
 . PMID 25852568. doi:10.3389/fphys.2015.00079.
. PMID 25852568. doi:10.3389/fphys.2015.00079. Aside from accounting for the reduced performance of mentally fatigued participants, this model rationalizes the reduced RPE and hence improved cycling time trial performance of athletes using a glucose mouthwash (Chambers et al., 2009) and the greater power output during a RPE matched cycling time trial following amphetamine ingestion (Swart, 2009). … Dopamine stimulating drugs are known to enhance aspects of exercise performance (Roelands et al., 2008)
- Jump up^ Roelands B, De Pauw K, Meeusen R (June 2015). “Neurophysiological effects of exercise in the heat”. Scand. J. Med. Sci. Sports. 25 Suppl 1: 65–78. PMID 25943657. doi:10.1111/sms.12350.
This indicates that subjects did not feel they were producing more power and consequently more heat. The authors concluded that the “safety switch” or the mechanisms existing in the body to prevent harmful effects are overridden by the drug administration (Roelands et al., 2008b). Taken together, these data indicate strong ergogenic effects of an increased DA concentration in the brain, without any change in the perception of effort.
- ^ Jump up to:a b c d e f g “Adderall XR Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. p. 11. Retrieved 30 December 2013.
- ^ Jump up to:a b c d e f g h i j k l “Adderall XR Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 4–8. Retrieved 30 December 2013.
- ^ Jump up to:a b c d “Vyvanse Prescribing Information” (PDF). Shire Inc. Retrieved 1 July 2014.
- ^ Jump up to:a b c d e f Heedes G; Ailakis J. “Amphetamine (PIM 934)”. INCHEM. International Programme on Chemical Safety. Retrieved 24 June 2014.
- ^ Jump up to:a b c d e f g “Adderall XR Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 4–6. Retrieved 30 December 2013.
- Jump up^ “FDA Pregnancy Categories” (PDF). United States Food and Drug Administration. 21 October 2004. Retrieved 31 October 2013.
- Jump up^ “Dexamphetamine tablets”. Therapeutic Goods Administration. Retrieved 12 April 2014.
- ^ Jump up to:a b Vitiello B (April 2008). “Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function”. Child Adolesc. Psychiatr. Clin. N. Am. 17 (2): 459–474. PMC 2408826
 . PMID 18295156. doi:10.1016/j.chc.2007.11.010.
. PMID 18295156. doi:10.1016/j.chc.2007.11.010. - Jump up^ Ramey JT, Bailen E, Lockey RF (2006). “Rhinitis medicamentosa” (PDF). J. Investig. Allergol. Clin. Immunol. 16 (3): 148–155. PMID 16784007. Retrieved 29 April 2015.
Table 2. Decongestants Causing Rhinitis Medicamentosa
– Nasal decongestants:
– Sympathomimetic:
• Amphetamine - ^ Jump up to:a b “FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in children and young adults”. United States Food and Drug Administration. 20 December 2011. Retrieved 4 November 2013.
- Jump up^ Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O’Duffy A, Connell FA, Ray WA (November 2011). “ADHD drugs and serious cardiovascular events in children and young adults”. N. Engl. J. Med. 365 (20): 1896–1904. PMC 4943074
 . PMID 22043968. doi:10.1056/NEJMoa1110212.
. PMID 22043968. doi:10.1056/NEJMoa1110212. - ^ Jump up to:a b “FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults”. United States Food and Drug Administration. 15 December 2011. Retrieved 4 November 2013.
- Jump up^ Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV (December 2011). “ADHD medications and risk of serious cardiovascular events in young and middle-aged adults”. JAMA. 306 (24): 2673–2683. PMC 3350308
 . PMID 22161946. doi:10.1001/jama.2011.1830.
. PMID 22161946. doi:10.1001/jama.2011.1830. - Jump up^ Montgomery KA (June 2008). “Sexual desire disorders”. Psychiatry (Edgmont). 5 (6): 50–55. PMC 2695750
 . PMID 19727285.
. PMID 19727285. - Jump up^ O’Connor PG (February 2012). “Amphetamines”. Merck Manual for Health Care Professionals. Merck. Retrieved 8 May 2012.
- ^ Jump up to:a b c d Shoptaw SJ, Kao U, Ling W (January 2009). Shoptaw SJ, Ali R, ed. “Treatment for amphetamine psychosis”. Cochrane Database Syst. Rev. (1): CD003026. PMID 19160215. doi:10.1002/14651858.CD003026.pub3.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention …
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) …
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis. - ^ Jump up to:a b Greydanus D. “Stimulant Misuse: Strategies to Manage a Growing Problem” (PDF). American College Health Association (Review Article). ACHA Professional Development Program. p. 20. Archived from the original (PDF) on 3 November 2013. Retrieved 2 November 2013.
- ^ Jump up to:a b Childs E, de Wit H (May 2009). “Amphetamine-induced place preference in humans”. Biol. Psychiatry. 65 (10): 900–904. PMC 2693956
 . PMID 19111278. doi:10.1016/j.biopsych.2008.11.016.
. PMID 19111278. doi:10.1016/j.biopsych.2008.11.016. This study demonstrates that humans, like nonhumans, prefer a place associated with amphetamine administration. These findings support the idea that subjective responses to a drug contribute to its ability to establish place conditioning.
- ^ Jump up to:a b Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 15: Reinforcement and Addictive Disorders”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–375. ISBN 9780071481274.
- ^ Jump up to:a b Spiller HA, Hays HL, Aleguas A (June 2013). “Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management”. CNS Drugs. 27 (7): 531–543. PMID 23757186. doi:10.1007/s40263-013-0084-8.
Amphetamine, dextroamphetamine, and methylphenidate act as substrates for the cellular monoamine transporter, especially the dopamine transporter (DAT) and less so the norepinephrine (NET) and serotonin transporter. The mechanism of toxicity is primarily related to excessive extracellular dopamine, norepinephrine, and serotonin.
- Jump up^ Collaborators (2015). “Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013” (PDF). Lancet. 385 (9963): 117–171. PMC 4340604
 . PMID 25530442. doi:10.1016/S0140-6736(14)61682-2. Retrieved 3 March 2015.
. PMID 25530442. doi:10.1016/S0140-6736(14)61682-2. Retrieved 3 March 2015. Amphetamine use disorders … 3,788 (3,425–4,145)
- Jump up^ Kanehisa Laboratories (10 October 2014). “Amphetamine – Homo sapiens (human)”. KEGG Pathway. Retrieved 31 October 2014.
- ^ Jump up to:a b c d e f Nechifor M (March 2008). “Magnesium in drug dependences”. Magnes. Res. 21 (1): 5–15. PMID 18557129.
- ^ Jump up to:a b c d e Ruffle JK (November 2014). “Molecular neurobiology of addiction: what’s all the (Δ)FosB about?”. Am. J. Drug Alcohol Abuse. 40 (6): 428–437. PMID 25083822. doi:10.3109/00952990.2014.933840.
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure.
- ^ Jump up to:a b c d e Nestler EJ (December 2013). “Cellular basis of memory for addiction”. Dialogues Clin. Neurosci. 15 (4): 431–443. PMC 3898681
 . PMID 24459410.
. PMID 24459410. Despite the importance of numerous psychosocial factors, at its core, drug addiction involves a biological process: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. … A large body of literature has demonstrated that such ΔFosB induction in D1-type [nucleus accumbens] neurons increases an animal’s sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement … Another ΔFosB target is cFos: as ΔFosB accumulates with repeated drug exposure it represses c-Fos and contributes to the molecular switch whereby ΔFosB is selectively induced in the chronic drug-treated state.41. … Moreover, there is increasing evidence that, despite a range of genetic risks for addiction across the population, exposure to sufficiently high doses of a drug for long periods of time can transform someone who has relatively lower genetic loading into an addict.
- Jump up^ Robison AJ, Nestler EJ (November 2011). “Transcriptional and epigenetic mechanisms of addiction”. Nat. Rev. Neurosci. 12 (11): 623–637. PMC 3272277
 . PMID 21989194. doi:10.1038/nrn3111.
. PMID 21989194. doi:10.1038/nrn3111. ΔFosB serves as one of the master control proteins governing this structural plasticity.
- ^ Jump up to:a b c d e f g h i j k l m n o p q r s t u v Olsen CM (December 2011). “Natural rewards, neuroplasticity, and non-drug addictions”. Neuropharmacology. 61 (7): 1109–1122. PMC 3139704
 . PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010.
. PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010. Similar to environmental enrichment, studies have found that exercise reduces self-administration and relapse to drugs of abuse (Cosgrove et al., 2002; Zlebnik et al., 2010). There is also some evidence that these preclinical findings translate to human populations, as exercise reduces withdrawal symptoms and relapse in abstinent smokers (Daniel et al., 2006; Prochaska et al., 2008), and one drug recovery program has seen success in participants that train for and compete in a marathon as part of the program (Butler, 2005). … In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al., 2006; Aiken, 2007; Lader, 2008).
- ^ Jump up to:a b c d Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA (September 2013). “Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis”. Neurosci. Biobehav. Rev. 37 (8): 1622–1644. PMC 3788047
 . PMID 23806439. doi:10.1016/j.neubiorev.2013.06.011.
. PMID 23806439. doi:10.1016/j.neubiorev.2013.06.011. These findings suggest that exercise may “magnitude”-dependently prevent the development of an addicted phenotype possibly by blocking/reversing behavioral and neuroadaptive changes that develop during and following extended access to the drug. … Exercise has been proposed as a treatment for drug addiction that may reduce drug craving and risk of relapse. Although few clinical studies have investigated the efficacy of exercise for preventing relapse, the few studies that have been conducted generally report a reduction in drug craving and better treatment outcomes … Taken together, these data suggest that the potential benefits of exercise during relapse, particularly for relapse to psychostimulants, may be mediated via chromatin remodeling and possibly lead to greater treatment outcomes.
- ^ Jump up to:a b c Zhou Y, Zhao M, Zhou C, Li R (July 2015). “Sex differences in drug addiction and response to exercise intervention: From human to animal studies”. Front. Neuroendocrinol. 40: 24–41. PMID 26182835. doi:10.1016/j.yfrne.2015.07.001.
Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. … The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation.
- ^ Jump up to:a b c Linke SE, Ussher M (January 2015). “Exercise-based treatments for substance use disorders: evidence, theory, and practicality”. Am. J. Drug Alcohol Abuse. 41 (1): 7–15. PMC 4831948
 . PMID 25397661. doi:10.3109/00952990.2014.976708.
. PMID 25397661. doi:10.3109/00952990.2014.976708. The limited research conducted suggests that exercise may be an effective adjunctive treatment for SUDs. In contrast to the scarce intervention trials to date, a relative abundance of literature on the theoretical and practical reasons supporting the investigation of this topic has been published. … numerous theoretical and practical reasons support exercise-based treatments for SUDs, including psychological, behavioral, neurobiological, nearly universal safety profile, and overall positive health effects.
- ^ Jump up to:a b Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 15: Reinforcement and Addictive Disorders”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 386. ISBN 9780071481274.
Currently, cognitive–behavioral therapies are the most successful treatment available for preventing the relapse of psychostimulant use.
- Jump up^ Greene SL, Kerr F, Braitberg G (October 2008). “Review article: amphetamines and related drugs of abuse”. Emerg. Med. Australas. 20 (5): 391–402. PMID 18973636. doi:10.1111/j.1742-6723.2008.01114.x.
- Jump up^ Albertson TE (2011). “Amphetamines”. In Olson KR, Anderson IB, Benowitz NL, Blanc PD, Kearney TE, Kim-Katz SY, Wu AH. Poisoning & Drug Overdose (6th ed.). New York: McGraw-Hill Medical. pp. 77–79. ISBN 9780071668330.
- Jump up^ “Glossary of Terms”. Mount Sinai School of Medicine. Department of Neuroscience. Retrieved 9 February 2015.
- Jump up^ Volkow ND, Koob GF, McLellan AT (January 2016). “Neurobiologic Advances from the Brain Disease Model of Addiction”. N. Engl. J. Med. 374 (4): 363–371. PMID 26816013. doi:10.1056/NEJMra1511480.
Substance-use disorder: A diagnostic term in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) referring to recurrent use of alcohol or other drugs that causes clinically and functionally significant impairment, such as health problems, disability, and failure to meet major responsibilities at work, school, or home. Depending on the level of severity, this disorder is classified as mild, moderate, or severe.
Addiction: A term used to indicate the most severe, chronic stage of substance-use disorder, in which there is a substantial loss of self-control, as indicated by compulsive drug taking despite the desire to stop taking the drug. In the DSM-5, the term addiction is synonymous with the classification of severe substance-use disorder. - Jump up^ Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 15: Reinforcement and Addictive Disorders”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 368. ISBN 9780071481274.
Such agents also have important therapeutic uses; cocaine, for example, is used as a local anesthetic (Chapter 2), and amphetamines and methylphenidate are used in low doses to treat attention deficit hyperactivity disorder and in higher doses to treat narcolepsy (Chapter 12). Despite their clinical uses, these drugs are strongly reinforcing, and their long-term use at high doses is linked with potential addiction, especially when they are rapidly administered or when high-potency forms are given.
- Jump up^ Kollins SH (May 2008). “A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders”. Curr. Med. Res. Opin. 24 (5): 1345–1357. PMID 18384709. doi:10.1185/030079908X280707.
When oral formulations of psychostimulants are used at recommended doses and frequencies, they are unlikely to yield effects consistent with abuse potential in patients with ADHD.
- Jump up^ Stolerman IP (2010). Stolerman IP, ed. Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. p. 78. ISBN 9783540686989.
- Jump up^ Coghill DR, Caballero B, Sorooshian S, Civil R (June 2014). “A systematic review of the safety of lisdexamfetamine dimesylate”. CNS Drugs. 28 (6): 497–511. PMC 4057639
 . PMID 24788672. doi:10.1007/s40263-014-0166-2.
. PMID 24788672. doi:10.1007/s40263-014-0166-2. The prodrug formulation of LDX may also lead to reduced abuse potential of LDX compared with immediate-release d-AMP.
- Jump up^ “Amphetamines: Drug Use and Abuse”. Merck Manual Home Edition. Merck. February 2003. Archived from the original on 17 February 2007. Retrieved 28 February 2007.
- Jump up^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M (September 2013). Pérez-Mañá C, ed. “Efficacy of psychostimulant drugs for amphetamine abuse or dependence”. Cochrane Database Syst. Rev. 9: CD009695. PMID 23996457. doi:10.1002/14651858.CD009695.pub2.
- Jump up^ Hyman SE, Malenka RC, Nestler EJ (July 2006). “Neural mechanisms of addiction: the role of reward-related learning and memory”. Annu. Rev. Neurosci. 29: 565–598. PMID 16776597. doi:10.1146/annurev.neuro.29.051605.113009.
- ^ Jump up to:a b c d e f g h Robison AJ, Nestler EJ (November 2011). “Transcriptional and epigenetic mechanisms of addiction”. Nat. Rev. Neurosci. 12 (11): 623–637. PMC 3272277
 . PLID 21989194. doi:10.1038/nrn3111.
. PLID 21989194. doi:10.1038/nrn3111. - ^ Jump up to:a b <` href=”https://en.wikipedia.org/wiki/Lisdexamfetamine#cite_ref-@ddiction_genetics_101-2″>c d <` href=”httpr://en.wikipedia.org/wiki/Lisdexamfetamine#cite_ref-Addiction_genetics_101-4″>e Rteiner H, Van Waes V (January 2013). “Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants”. Prog. Neurobiol. 100: 60–80. PMC 3525776
 . PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001.
. PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001. - Jump up^ Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 4: Signal Transduction in the Brain”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 94. ISBN 9780071481274.
- Jump up^ Kanehisa Laboratories (29 October 2014). “Alcoholism – Homo sapiens (human)”. KEGG Pathway. Retrieved 31 October 2014.
- Jump up^ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (February 2009). “Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens”. Proc. Natl. Acad. Sci. U.S.A. 106 (8): 2915–2920. PMC 2650365
 . PMID 19202072. doi:10.1073/pnas.0813179106.
. PMID 19202072. doi:10.1073/pnas.0813179106. - Jump up^ Nestler EJ (January 2014). “Epigenetic mechanisms of drug addiction”. Neuropharmacology. 76 Pt B: 259–268. PMC 3766384
 . PMID 23643695. doi:10.1016/j.neuropharm.2013.04.004.
. PMID 23643695. doi:10.1016/j.neuropharm.2013.04.004. - ^ Jump up to:a b Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M (March 2012). “Sex, drugs, and rock ‘n’ roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms”. J. Psychoactive Drugs. 44 (1): 38–55. PMC 4040958
 . PMID 22641964. doi:10.1080/02791072.2012.662112.
. PMID 22641964. doi:10.1080/02791072.2012.662112. - Jump up^ Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (February 2013). “Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator”. J. Neurosci. 33 (8): 3434–3442. PMC 3865508
 . PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013.
. PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013. - Jump up^ Beloate LN, Weems PW, Casey GR, Webb IC, Coolen LM (February 2016). “Nucleus accumbens NMDA receptor activation regulates amphetamine cross-sensitization and deltaFosB expression following sexual experience in male rats”. Neuropharmacology. 101: 154–164. PMID 26391065. doi:10.1016/j.neuropharm.2015.09.023.
- Jump up^ Stoops WW, Rush CR (May 2014). “Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research”. Expert Rev Clin Pharmacol. 7 (3): 363–374. PMC 4017926
 . PMID 24716825. doi:10.1586/17512433.2014.909283.
. PMID 24716825. doi:10.1586/17512433.2014.909283. Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved.
- Jump up^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M (September 2013). “Efficacy of psychostimulant drugs for amphetamine abuse or dependence”. Cochrane Database Syst. Rev. 9: CD009695. PMID 23996457. doi:10.1002/14651858.CD009695.pub2.
To date, no pharmacological treatment has been approved for [addiction], and psychotherapy remains the mainstay of treatment. … Results of this review do not support the use of psychostimulant medications at the tested doses as a replacement therapy
- Jump up^ Forray A, Sofuoglu M (February 2014). “Future pharmacological treatments for substance use disorders”. Br. J. Clin. Pharmacol. 77 (2): 382–400. PMC 4014020
 . PMID 23039267. doi:10.1111/j.1365-2125.2012.04474.x.
. PMID 23039267. doi:10.1111/j.1365-2125.2012.04474.x. - ^ Jump up to:a b Grandy DK, Miller GM, Li JX (February 2016). “”TAARgeting Addiction”-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference”. Drug Alcohol Depend. 159: 9–16. PMID 26644139. doi:10.1016/j.drugalcdep.2015.11.014.
When considered together with the rapidly growing literature in the field a compelling case emerges in support of developing TAAR1-selective agonists as medications for preventing relapse to psychostimulant abuse.
- ^ Jump up to:a b Jing L, Li JX (August 2015). “Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction”. Eur. J. Pharmacol. 761: 345–352. PMC 4532615
 . PMID 26092759. doi:10.1016/j.ejphar.2015.06.019.
. PMID 26092759. doi:10.1016/j.ejphar.2015.06.019. Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction.
- ^ Jump up to:a b Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 5: Excitatory and Inhibitory Amino Acids”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 124–125. ISBN 9780071481274.
- ^ Jump up to:a b c Carroll ME, Smethells JR (February 2016). “Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments”. Front. Psychiatry. 6: 175. PMC 4745113
 . PMID 26903885. doi:10.3389/fpsyt.2015.00175.
. PMID 26903885. doi:10.3389/fpsyt.2015.00175. Physical Exercise
There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction … In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. … The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in [laboratory animals and humans] regarding exercise as a treatment for drug addiction supports this hypothesis. … Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon. - ^ Jump up to:a b c d Shoptaw SJ, Kao U, Heinzerling K, Ling W (April 2009). Shoptaw SJ, ed. “Treatment for amphetamine withdrawal”. Cochrane Database Syst. Rev. (2): CD003021. PMID 19370579. doi:10.1002/14651858.CD003021.pub2.
- Jump up^ “Dexedrine Prescribing Information” (PDF). United States Food and Drug Administration. Amedra Pharmaceuticals LLC. October 2013. Retrieved 4 November 2013.
- Jump up^ “Adderall IR Prescribing Information” (PDF). United States Food and Drug Administration. Teva Pharmaceuticals USA, Inc. October 2015. Retrieved 18 May 2016.
- Jump up^ “Adderall XR Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. Retrieved 30 December 2013.
- Jump up^ Advokat C (July 2007). “Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD”. J. Atten. Disord. 11 (1): 8–16. PMID 17606768. doi:10.1177/1087054706295605.
- ^ Jump up to:a b c d Bowyer JF, Hanig JP (November 2014). “Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity”. Temperature (Austin). 1 (3): 172–182. PMC 5008711
 . PMID 27626044. doi:10.4161/23328940.2014.982049.
. PMID 27626044. doi:10.4161/23328940.2014.982049. Hyperthermia alone does not produce amphetamine-like neurotoxicity but AMPH and METH exposures that do not produce hyperthermia (≥40°C) are minimally neurotoxic. Hyperthermia likely enhances AMPH and METH neurotoxicity directly through disruption of protein function, ion channels and enhanced ROS production. … The hyperthermia and the hypertension produced by high doses amphetamines are a primary cause of transient breakdowns in the blood-brain barrier (BBB) resulting in concomitant regional neurodegeneration and neuroinflammation in laboratory animals. … In animal models that evaluate the neurotoxicity of AMPH and METH, it is quite clear that hyperthermia is one of the essential components necessary for the production of histological signs of dopamine terminal damage and neurodegeneration in cortex, striatum, thalamus and hippocampus.
- Jump up^ “Amphetamine”. Hazardous Substances Data Bank. United States National Library of Medicine – Toxicology Data Network. Retrieved 26 February 2014.
Direct toxic damage to vessels seems unlikely because of the dilution that occurs before the drug reaches the cerebral circulation.
- Jump up^ Malenka RC, Nestler EJ, Hyman SE (2009). “Chapter 15: Reinforcement and addictive disorders”. In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
- Jump up^ Sulzer D, Zecca L (February 2000). “Intraneuronal dopamine-quinone synthesis: a review”. Neurotox. Res. 1 (3): 181–195. PMID 12835101. doi:10.1007/BF03033289.
- Jump up^ Miyazaki I, Asanuma M (June 2008). “Dopaminergic neuron-specific oxidative stress caused by dopamine itself” (PDF). Acta Med. Okayama. 62 (3): 141–150. PMID 18596830.
- Jump up^ Hofmann FG (1983). A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects (2nd ed.). New York, USA: Oxford University Press. p. 329. ISBN 9780195030570.
- ^ Jump up to:a b c d “Adderall XR Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 8–10. Retrieved 30 December 2013.
- ^ Jump up to:a b “Identification”. Lisdexamfetamine. DrugBank. University of Alberta. 16 September 2013. Retrieved 13 June 2014.
- ^ Jump up to:a b c Jasinski DR, Krishnan S (June 2009). “Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse”. J. Psychopharmacol. (Oxford). 23 (4): 419–427. PMID 19329547. doi:10.1177/0269881109103113.
- ^ Jump up to:a b Miller GM (January 2011). “The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity”. J. Neurochem. 116 (2): 164–176. PMC 3005101
 . PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x.
. PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x. - ^ Jump up to:a b Eiden LE, Weihe E (January 2011). “VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse”. Ann. N. Y. Acad. Sci. 1216: 86–98. Bibcode:2011NYASA1216…86E. PMC 4183197
 . PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x.
. PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x. VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) … [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).
- Jump up^ “Adderall XR Prescribing Information” (PDF). United States Food and Drug Administration. pp. 1–18. Retrieved 7 October 2013.
- Jump up^ “Pharmacology”. Dextroamphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 5 November 2013.
- ^ Jump up to:a b c d e f g h i j k l m n “Adderall XR Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- Jump up^ “Pharmacology”. Amphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 5 November 2013.
- ^ Jump up to:a b c d “Pharmacology and Biochemistry”. Amphetamine. Pubchem Compound. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 12 October 2013.
- ^ Jump up to:a b “Metabolism/Pharmacokinetics”. AMPHETAMINE. United States National Library of Medicine – Toxicology Data Network. Hazardous Substances Data Bank. Retrieved 5 January 2014.
Plasma protein binding, rate of absorption, & volumes of distribution of amphetamine isomers are similar. … The biological half-life of amphetamine is greater in drug dependent individuals than in control subjects, & distribution volumes are increased, indicating that greater affinity of tissues for the drug may contribute to development of amphetamine tolerance. … Concentrations of (14)C-amphetamine declined less rapidly in the plasma of human subjects maintained on an alkaline diet (urinary pH > 7.5) than those on an acid diet (urinary pH < 6). Plasma half-lives of amphetamine ranged between 16-31 hr & 8-11 hr, respectively, & the excretion of (14)C in 24 hr urine was 45 & 70%.
- ^ Jump up to:a b c “Vyvanse Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. January 2017. pp. 18–21. Retrieved 16 February 2017.
- Jump up^ Glennon RA (2013). “Phenylisopropylamine stimulants: amphetamine-related agents”. In Lemke TL, Williams DA, Roche VF, Zito W. Foye’s principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). … The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. … Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. … Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- Jump up^ Taylor KB (January 1974). “Dopamine-beta-hydroxylase. Stereochemical course of the reaction” (PDF). J. Biol. Chem. 249 (2): 454–458. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- Jump up^ Krueger SK, Williams DE (June 2005). “Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism”. Pharmacol. Ther. 106 (3): 357–387. PMC 1828602
 . PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
. PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - Jump up^ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). “N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication”. J. Pharmacol. Exp. Ther. 288 (3): 1251–1260. PMID 10027866.
- ^ Jump up to:a b c Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). “Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection”. J. Pharm. Biomed. Anal. 30 (2): 247–255. PMID 12191709. doi:10.1016/S0731-7085(02)00330-8.
- Jump up^ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). “Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity”. Circ. Res. 32 (5): 594–599. PMID 4713201. doi:10.1161/01.RES.32.5.594.
Subjects with exceptionally low levels of serum dopamine-β-hydroxylase activity showed normal cardiovascular function and normal β-hydroxylation of an administered synthetic substrate, hydroxyamphetamine.
- ^ Jump up to:a b “Substrate/Product”. butyrate-CoA ligase. BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014.
- ^ Jump up to:a b “Substrate/Product”. glycine N-acyltransferase. BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014.
- Jump up^ “Compound Summary”. p-Hydroxyamphetamine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- Jump up^ “Compound Summary”. p-Hydroxynorephedrine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- Jump up^ “Compound Summary”. Phenylpropanolamine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- Jump up^ “Molecular Weight Calculator”. Lenntech. Retrieved 19 August 2015.
- ^ Jump up to:a b “Dextroamphetamine Sulfate USP”. Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ^ Jump up to:a b “D-amphetamine sulfate”. Tocris. 2015. Retrieved 19 August 2015.
- ^ Jump up to:a b “Amphetamine Sulfate USP”. Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- Jump up^ “Dextroamphetamine Saccharate”. Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- Jump up^ “Amphetamine Aspartate”. Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- Jump up^ “Vyvanse Prescribing Information” (PDF). United States Food and Drug Administration. Shire US Inc. January 2015. pp. 12–16. Retrieved 24 February 2015.
- Jump up^ Lisdexamfetamine Dimesylate: A Prodrug Stimulant for the Treatment of ADHD in Children and Adults
- Jump up^ FDA Adult Approval of Vyvanse – FDA Label and Approval History
- Jump up^ Health Canada Notice of Compliance – Vyvanse. 19 February 2009, retrieved on 9 March 2009.
- Jump up^ [1]. 8 February 2012, retrieved on 9 February 2012.
- Jump up^ Hirschler, Ben (7 February 2014). “UPDATE 2-Shire scraps Vyvanse for depression after failed trials”. Reuters. Retrieved 13 February 2014.
- Jump up^ http://www.shire.com/shireplc/en/investors/irshirenews?id=684
- Jump up^ http://www.shire.com/shireplc/en/investors/investorsnews/irshirenews?id=1055
- Jump up^ http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm432543.htm
- Jump up^ Cassels, Caroline. “FDA Okays Vyvanse for Binge Eating Disorder”. medscape.com. Retrieved 30 January 2015.
- Jump up^ “Drugs@FDA: FDA Approved Drug Products”. www.accessdata.fda.gov. Retrieved 14 February 2017.
- Jump up^ http://www.shire.com/shireplc/en/investors/investorsnews/irshirenews?id=684
- Jump up^ Dale E, Bang-Andersen B, Sánchez C (May 2015). “Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs”. Biochem. Pharmacol. 95 (2): 81–97. PMID 25813654. doi:10.1016/j.bcp.2015.03.011.
- Jump up^ “Psychostimulants in the therapy of treatment-resistant depression Review of the literature and findings from a retrospective study in 65 depressed patients”. Dialogues Clin Neurosci. 1: 165–74. 1999. PMC 3181580
 . PMID 22034135.
. PMID 22034135.
////////
CC(CC1=CC=CC=C1)NC(=O)C(CCCCN)N