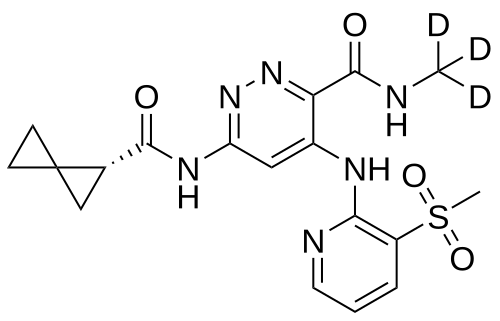
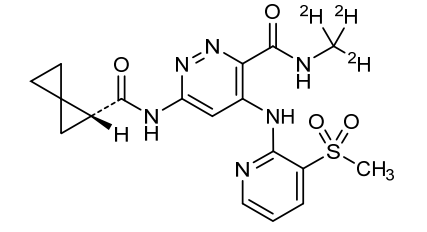
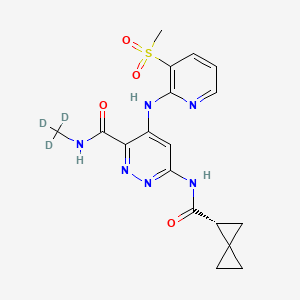
Lomedeucitinib
CAS 2328068-29-5
MF C18H172H3N6O4S
MW 419.5 g/mol
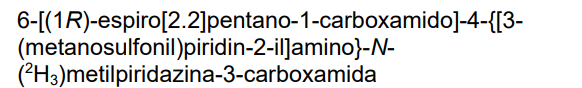
4-{[3-(methanesulfonyl)pyridin-2-yl]amino}-N-(2H3)methyl-6-[(1R)-spiro[2.2]pentane-1-carboxamido]pyridazine-3-carboxamide
4-[(3-methylsulfonyl-2-pyridinyl)amino]-6-[[(2R)-spiro[2.2]pentane-2-carbonyl]amino]-N-(trideuteriomethyl)pyridazine-3-carboxamide
Janus kinase inhibitor, anti-inflammatory, BMS-986322, BMS 986322, EYQ7KA55XA
Lomedeucitinib is an investigational new drug that is being evaluated for the treatment of psoriasis and psoriatic arthritis. It is a tyrosine kinase 2 (TYK2) inhibitor.[1]
- A Study to Evaluate Effectiveness and Safety of BMS-986322 in Participants With Moderate-to-Severe PsoriasisCTID: NCT05730725Phase: Phase 2Status: CompletedDate: 2024-09-19
- A Study to Evaluate the Drug Levels, Metabolism, and Removal of BMS-986322 in Healthy Adult Male ParticipantsCTID: NCT06088264Phase: Phase 1Status: CompletedDate: 2024-03-29
- A Study Investigating Interactions Between BMS-986322 and Rosuvastatin, Metformin and Methotrexate in Healthy ParticipantsCTID: NCT05615012Phase: Phase 1Status: CompletedDate: 2024-03-27
- A Study to Investigate the Interaction of BMS-986322 and a Combined Oral Hormonal Contraceptive (Ethinyl Estradiol [EE]/Norethindrone [NET]) in Healthy Female ParticipantsCTID: NCT05579574Phase: Phase 1Status: CompletedDate: 2023-08-18
- A Study to Assess the Safety and Tolerability of BMS-986322 in Healthy Participants of Japanese DescentCTID: NCT05546151Phase: Phase 1Status: CompletedDate: 2023-06-22
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US333829535&_cid=P10-MHIXWK-98212-1
General Scheme for Examples 252 and 253:
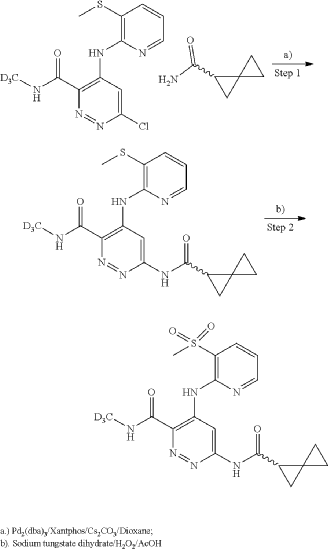
Example 252
Step 1
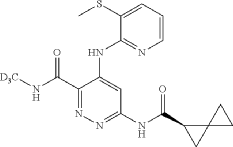
| A mixture of cesium carbonate (149 mg, 0.457 mmol), Xantphos (14.43 mg, 0.025 mmol), Pd 2(dba) 3 (11.42 mg, 0.012 mmol), 6-chloro-N-(methyl-d3)-4-((3-(methylthio)pyridin-2-yl)amino)pyridazine-3-carboxamide (65 mg, 0.208 mmol), and (R)-spiro[2.2]pentane-1-carboxamide (50.8 mg, 0.457 mmol) in dioxane (3 mL) was degassed using a vacuum/N2 fill cycle three times. The reaction was heated at 110° C. for 16 hours. The reaction was diluted with water and DCM. The DCM layer was separated and washed two more times with water and then dried (Na 2SO 4), filtered and concentrated. Purification via automated flash chromatography, eluting with methanol in DCM from 0 to 10%, gave the title compound (R)—N-(methyl-d3)-4-((3-(methylthio)pyridin-2-yl)amino)-6-(spiro[2.2]pentane-1-carboxamido)pyridazine-3-carboxamide (54 mg, 67% yield). 1H NMR (400 MHz, CHLOROFORM-d) δ 12.15 (br s, 1H), 9.88 (s, 1H), 8.68 (br s, 1H), 8.36 (br d, J=3.5 Hz, 1H), 8.25 (br s, 1H), 7.72 (br d, J=7.4 Hz, 1H), 6.97 (br dd, J=7.0, 5.1 Hz, 1H), 2.51 (s, 3H), 2.21-2.09 (m, 1H), 1.58-1.10 (m, 6H), 1.08-0.93 (m, 5H). |
| LCMS (ESI) m/e 388.1 [(M+H) +, calc’d C 18H 18D 3N 6O 2S 1, 388.1]; LC/MS retention time (method D): t R=0.80 min. |
Step 2
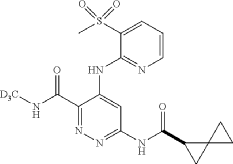
To a suspension of hydrogen peroxide (30% solution in water, 0.258 mL, 2.52 mmol) and (R)—N-(methyl-d3)-4-((3-(methylthio)pyridin-2-yl)amino)-6-(spiro[2.2]pentane-1-carboxamido)pyridazine-3-carboxamide (0.0489 g, 0.126 mmol) in AcOH (1 mL) was added sodium tungstate dihydrate (0.042 g, 0.126 mmol) at room temperature. After stirring at room temperature for 1 hour, the reaction was diluted with water, basified with Na 2CO 3 powder and extracted three times with DCM. The DCM layers were combined, washed with Na 2S 2O 3 (5% solution), dried (Na 2SO 4), filtered and concentrated. The crude product was purified using reverse phase prepHPLC to give the title compound (R)—N-(methyl-d3)-4-((3-(methylsulfonyl)pyridin-2-yl)amino)-6-(spiro[2.2]pentane-1-carboxamido)pyridazine-3-carboxamide (16.2 mg, 31%) as a colorless solid. 1H NMR (500 MHz, DMSO-d 6) δ 12.07 (s, 1H), 11.22 (s, 1H), 9.49 (s, 1H), 9.16 (s, 1H), 8.63 (dd, J=4.6, 1.5 Hz, 1H), 8.29 (dd, 0.1=7.8, 1.4 Hz, 1H), 7.34 (dd, 0.1=7.8, 4.7 Hz, 1H), 2.48-2.43 (m, 1H), 1.46-1.41 (m, 1H), 1.42-1.36 (m, 1H), 0.95-0.82 (m, 3H), 0.80-0.73 (m, 1H). (3H methyl sulfone was buried under DMSO peak). LCMS (ESI) m/e 420.0 [(M+H) +, calc’d C 18H 18D 3N 6O 4S, 420.1]; LC/MS retention time (method E): t R=1.38 min; OR: −205.39 (20° C.).
SYN
https://patentscope.wipo.int/search/en/detail.jsf?docId=US242383764&_cid=P10-MHIXVD-97150-1
PAT
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: US-11787779-B2Priority Date: 2017-11-21Grant Date: 2023-10-17
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: US-2024002364-A1Priority Date: 2017-11-21
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: KR-102702228-B1Priority Date: 2017-11-21Grant Date: 2024-09-02
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: NZ-805343-APriority Date: 2017-11-21
- Sulfonepyridine alkylamide-substituted heteroaryl compoundsPublication Number: JP-2023098942-APriority Date: 2017-11-21
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: AU-2023255024-A1Priority Date: 2017-11-21
- Heteroaryl compounds substituted with sulfone pyridinylalkylamidesPublication Number: CN-111315737-BPriority Date: 2017-11-21Grant Date: 2024-06-18
- The heteroaryl compounds are substituted with sulfone-pyridine alkyl amidesPublication Number: IL-274816-B2Priority Date: 2017-11-21
- Sulfonepyridine alkylamide substituted heteroaryl compoundsPublication Number: JP-7490107-B2Priority Date: 2017-11-21Grant Date: 2024-05-24
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: AU-2022228101-A1Priority Date: 2017-11-21
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: TW-I776994-BPriority Date: 2017-11-21Grant Date: 2022-09-11
- Sulfonepyridine alkylamide-substituted heteroaryl compoundsPublication Number: JP-7258903-B2Priority Date: 2017-11-21Grant Date: 2023-04-17
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: AU-2022228101-B2Priority Date: 2017-11-21Grant Date: 2023-08-03
- The heteroaryl compounds are substituted with sulfone-pyridine alkyl amidesPublication Number: IL-274816-B1Priority Date: 2017-11-21
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: US-2019152948-A1Priority Date: 2017-11-21
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: CA-3083122-A1Priority Date: 2017-11-21
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: KR-20200089706-APriority Date: 2017-11-21
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: US-11021462-B2Priority Date: 2017-11-21Grant Date: 2021-06-01
- Sulfone pyridine alkyl amide-substituted heteroaryl compoundsPublication Number: US-2021253554-A1Priority Date: 2017-11-21



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
| Clinical data | |
|---|---|
| Other names | BMS-986322 |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2328068-29-5 |
| PubChem CID | 138620496 |
| IUPHAR/BPS | 13210 |
| UNII | EYQ7KA55XA |
| KEGG | D12725 |
| ChEMBL | ChEMBL5314608 |
| Chemical and physical data | |
| Formula | C18H17D3N6O4S |
| Molar mass | 419.47 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
References
- Ahsan S, Degener R, Schlamp M (2024). “Non-Invasive Treatments Invade the Psoriasis Pipeline”. Drugs in Context. 13: 2024–5–6. doi:10.7573/dic.2024-5-6. PMC 11313207. PMID 39131603.
////////lomedeucitinib, Janus kinase inhibitor, anti-inflammatory, BMS-986322, BMS 986322, EYQ7KA55XA














