
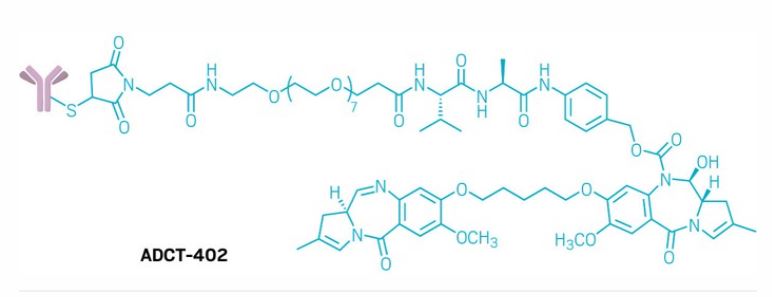
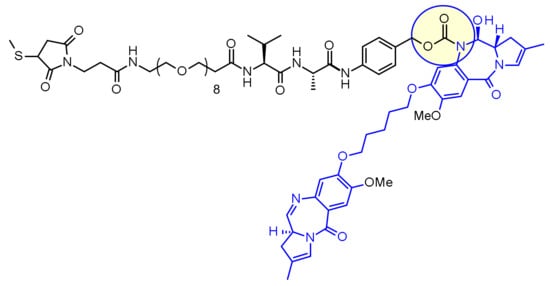
Loncastuximab tesirine
ZYNLONTA FDA APPROVED 2021/4/23
| Formula |
C6544H10048N1718O2064S52
|
|---|---|
| Exact mass |
147387.9585
|
| CAS |
1879918-31-6
|
| Efficacy |
Antineoplasitc, Anti-CD19 antibody
|
| Disease |
Diffuse large B-cell lymphoma not otherwise specified [DS:H02434]
|
| Comment |
Antibody-drug conjugate
Treatment of hematological cancers |
ロンカスツキシマブテシリン; ADCT-402, ADCX 19
Immunoglobulin G1, anti-(human CD19 antigen) (human-Mus musculus monoclonal RB4v1.2 γ1-chain), disulfide with human-Mus musculus monoclonal RB4v1.2 κ-chain, dimer, bis(thioether) with N-[31-(3-mercapt-2,5-dioxo-1-pyrrolidinyl)-1,29-dioxo-4,7,10,13,16,19,22,25-octaoxa-28-azahentriacont-1-yl]-L-valyl-N-[4-[[[[(11S,11aS)-8-[[5-[[(11aS)-5,11a-dihydro-7-methoxy-2-methyl-5-oxo-1H-pyrrolo[2,1-c][1,4]benzodiazepin-8-yl]oxy]pentyl]oxy]-11,11a-dihydro-11-hydroxy-7-methoxy-2-methyl-5-oxo-1H-pyrrolo[2,1-c][1,4]benzodiazepin-10(5H)-yl]carbonyl]oxy]methyl]phenyl]-L-alaninamide
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | CD19 |
| Clinical data | |
| Trade names | Zynlonta |
| Other names | ADCT-402, loncastuximab tesirine-lpyl |
| License data | |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number |
|
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6544H10048N1718O2064S52 |
| Molar mass | 147481.45 g·mol−1 |
Loncastuximab tesirine-lpyl is a CD19-directed antibody and alkylating agent conjugate, consisting of a humanized IgG1 kappa monoclonal antibody conjugated to SG3199, a pyrrolobenzodiazepine (PBD) dimer cytotoxic alkylating agent, through a protease-cleavable valine–alanine linker. SG3199 attached to the linker is designated as SG3249, also known as tesirine.
 |
Loncastuximab tesirine-lpyl has an approximate molecular weight of 151 kDa. An average of 2.3 molecules of SG3249 are attached to each antibody molecule. Loncastuximab tesirine-lpyl is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells, and the small molecule components are produced by chemical synthesis.
ZYNLONTA (loncastuximab tesirine-lpyl) for injection is supplied as a sterile, white to off-white, preservative-free, lyophilized powder, which has a cake-like appearance, for intravenous infusion after reconstitution and dilution. Each single-dose vial delivers 10 mg of loncastuximab tesirine-lpyl, L-histidine (2.8 mg), L-histidine monohydrochloride (4.6 mg), polysorbate 20 (0.4 mg), and sucrose (119.8 mg). After reconstitution with 2.2 mL Sterile Water for Injection, USP, the final concentration is 5 mg/mL with a pH of approximately 6.0.
Loncastuximab tesirine , sold under the brand name Zynlonta, is used for the treatment of large B-cell lymphoma. It is an antibody-drug conjugate (ADC) composed of a humanized antibody targeting the protein CD19, which is expressed in a wide range of B cell hematological tumors.[2] The experimental drug, developed by ADC Therapeutics is being tested in clinical trials for the treatment of B-cell non-Hodgkin lymphoma (NHL) and B-cell acute lymphoblastic leukemia (ALL).
On April 23, 2021, the Food and Drug Administration granted accelerated approval to loncastuximab tesirine-lpyl (Zynlonta, ADC Therapeutics SA), a CD19-directed antibody and alkylating agent conjugate, for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma.
Approval was based on LOTIS-2 (NCT03589469), an open-label, single-arm trial in 145 adult patients with relapsed or refractory DLBCL or high-grade B-cell lymphoma after at least two prior systemic regimens. Patients received loncastuximab tesirine-lpyl 0.15 mg/kg every 3 weeks for 2 cycles, then 0.075 mg/kg every 3 weeks for subsequent cycles. Patients received treatment until progressive disease or unacceptable toxicity.
The main efficacy outcome measure was overall response rate (ORR), as assessed by an independent review committee using Lugano 2014 criteria. The ORR was 48.3% (95% CI: 39.9, 56.7) with a complete response rate of 24.1% (95% CI: 17.4, 31.9). After a median follow-up of 7.3 months, median response duration was 10.3 months (95% CI: 6.9, NE). Of the 70 patients who achieved objective responses, 36% were censored for response duration prior to 3 months.
Most common (≥20%) adverse reactions in patients receiving loncastuximab tesirine-lpyl, including laboratory abnormalities, are thrombocytopenia, increased gamma-glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
The prescribing information provides warnings and precautions for adverse reactions including edema and effusions, myelosuppression, infections, and cutaneous reactions.
The recommended loncastuximab tesirine-lpyl dosage is 0.15 mg/kg every 3 weeks for 2 cycles, then 0.075 mg/kg every 3 weeks for subsequent cycles, by intravenous infusion over 30 minutes on day 1 of each cycle (every 3 weeks). Patients should be premedicated with dexamethasone 4 mg orally or intravenously twice daily for 3 days beginning the day before loncastuximab tesirine-lpyl.
Technology
The humanized monoclonal antibody is stochastically conjugated via a valine-alanine cleavable, maleimide linker to a cytotoxic (anticancer) pyrrolobenzodiazepine (PBD) dimer. The antibody binds to CD19, a protein which is highly expressed on the surface of B-cell hematological tumors[3] including certain forms of lymphomas and leukemias. After binding to the tumor cells the antibody is internalized, the cytotoxic drug PBD is released and the cancer cells are killed. PBD dimers are generated out of PBD monomers, a class of natural products produced by various actinomycetes. PBD dimers work by crosslinking specific sites of the DNA, blocking the cancer cells’ division that cause the cells to die. As a class of DNA-crosslinking agents they are significantly more potent than systemic chemotherapeutic drugs.[4]
Clinical trials
Two phase I trials are evaluating the drug in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma and relapsed or refractory B-cell acute lymphoblastic leukemia.[5] At the 14th International Conference on Malignant Lymphoma interim results from a Phase I, open-label, dose-escalating study designed to evaluate the treatment of loncastuximab tesirine in relapsed or refractory non-Hodgkin’s lymphoma were presented.[6] Among the patients enrolled at the time of the data cutoff the overall response rate was 61% in the total patient population (42% complete response and 19% partial response) and in patients with relapsing or refractory diffuse large B-cell lymphoma (DLBCL) the overall response rate was 57% (43% complete response and 14% partial response).[7][8]
Orphan drug designation
Loncastuximab tesirine was granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) for the treatment of diffuse large B-cell lymphoma and mantle cell lymphoma.[9]
References
- ^ https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761196s000lbl.pdf
- ^ WHO Drug Information: International Nonproprietary Names for Pharmaceutical Substances
- ^ Wang K, Wei G, Liu D (November 2012). “CD19: a biomarker for B cell development, lymphoma diagnosis and therapy”. Experimental Hematology & Oncology. 1 (1): 36. doi:10.1186/2162-3619-1-36. PMC 3520838. PMID 23210908.
- ^ “Pyrrolobenzodiazepine”. ADC Review.
- ^ Clinical trial number NCT02669017 for “ADCT-402 in B-NHL” at ClinicalTrials.gov
- ^ Kahl B, Hamadani M, Caimi PF, Reid EG, Havenith K, He S, Feingold JM, O’Connor O (June 2017). “First clinical results of ADCT‐402, a novel pyrrolobenzodiazepine-based antibody drug conjugate (ADC), in relapsed/refractory B‐cell linage NHL” (PDF). Hematol Oncol. 35 (S2): 49–51. doi:10.1002/hon.2437_33.
- ^ “First clinical results of ADCT-402”. ADC Review.
- ^ Bainbridge K. “Grandfather fighting deadly cancer reveals scans of tumors after testing new drug”. Mirror.
- ^ “ADCT-402 Orphan Drug Designation” (PDF). ADC Therapeutics press release.
External links
- “Loncastuximab tesirine”. Drug Information Portal. U.S. National Library of Medicine.
/////////Loncastuximab tesirine, FDA 2021, APPROVALS 2021, ZYNLONTA, ロンカスツキシマブテシリン, ORPHAN DRUG, ADCT-402, priority review, ADCX 19














