
LOXO-195
CAS: 2097002-61-2
Chemical Formula: C20H21FN6O
Molecular Weight: 380.4274
2097002-59-8 (RS-isomer) 1350884-56-8 (R racemic)
Synonym: LOXO-195; LOXO 195; LOXO195.
IUPAC: (13E,14E,22R,6R)-35-fluoro-6-methyl-7-aza-1(5,3)-pyrazolo[1,5-a]pyrimidina-3(3,2)-pyridina-2(1,2)-pyrrolidinacyclooctaphan-8-one
10H-5,7-Ethenopyrazolo[3,4-d]pyrido[2,3-k]pyrrolo[2,1-m][1,3,7]triazacyclotridecin-10-one, 17-fluoro-1,2,3,11,12,13,14,18b-octahydro-12-methyl-, (12R,18bR)-
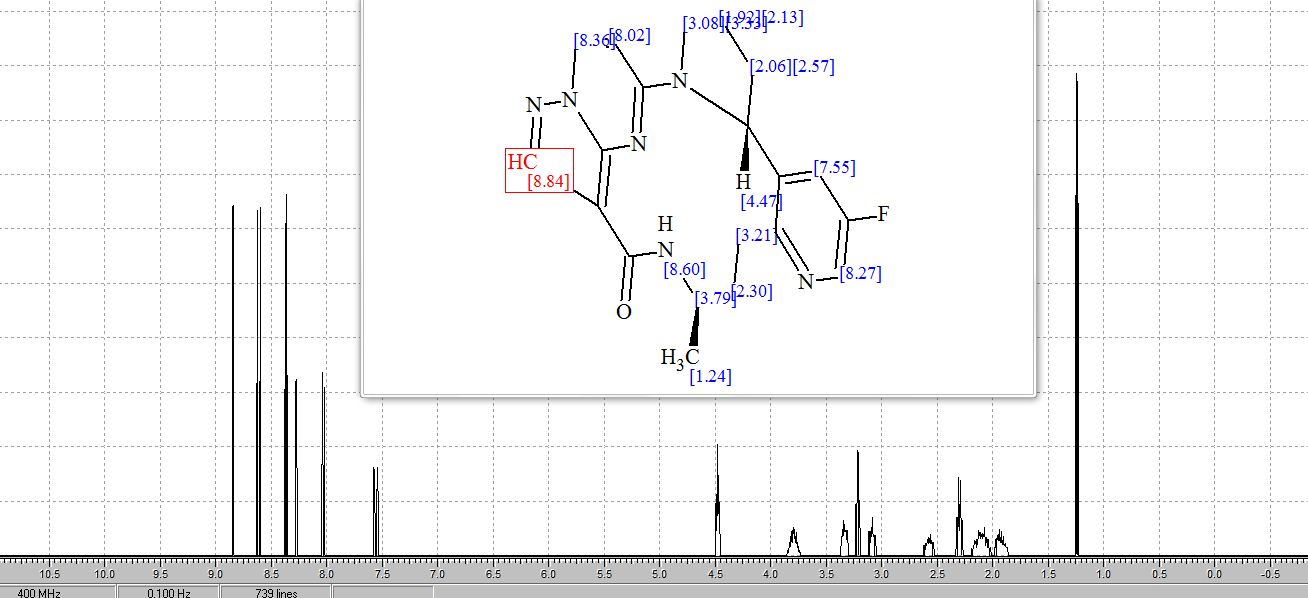
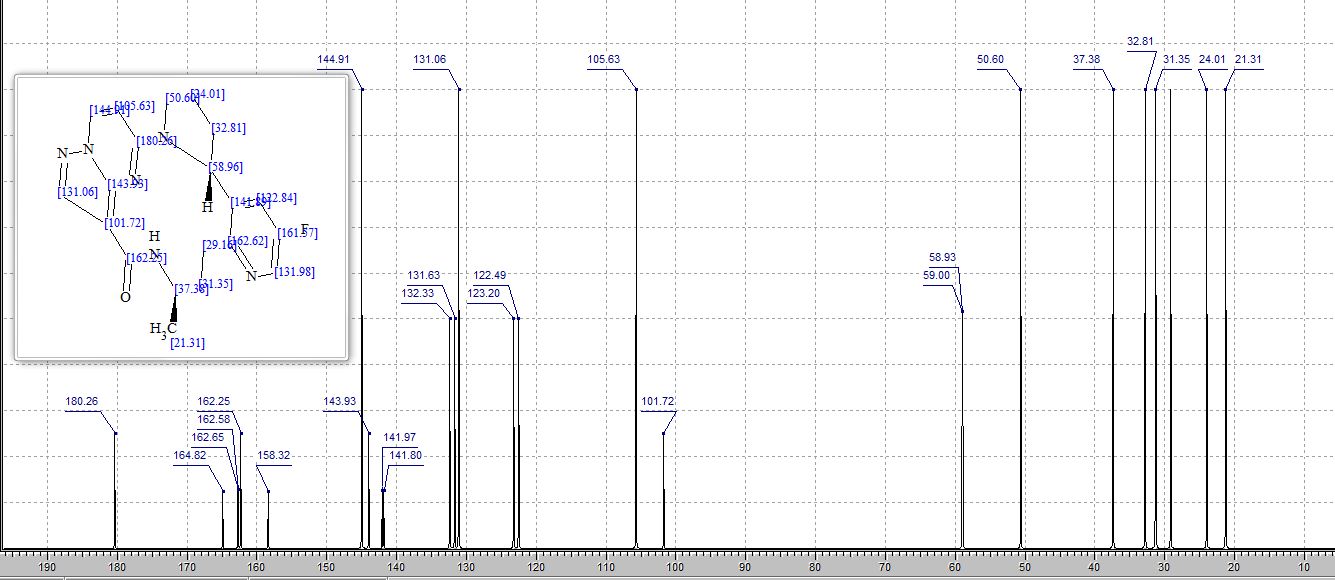
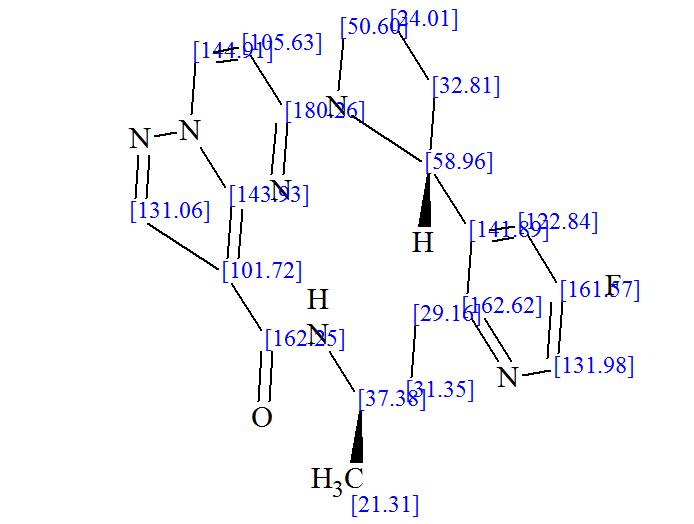
SMILES Code: FC1=CN=C(CC[C@@H](C)NC(C2=C3N(C=CC4=N3)N=C2)=O)C([C@@H]5N4CCC5)=C1

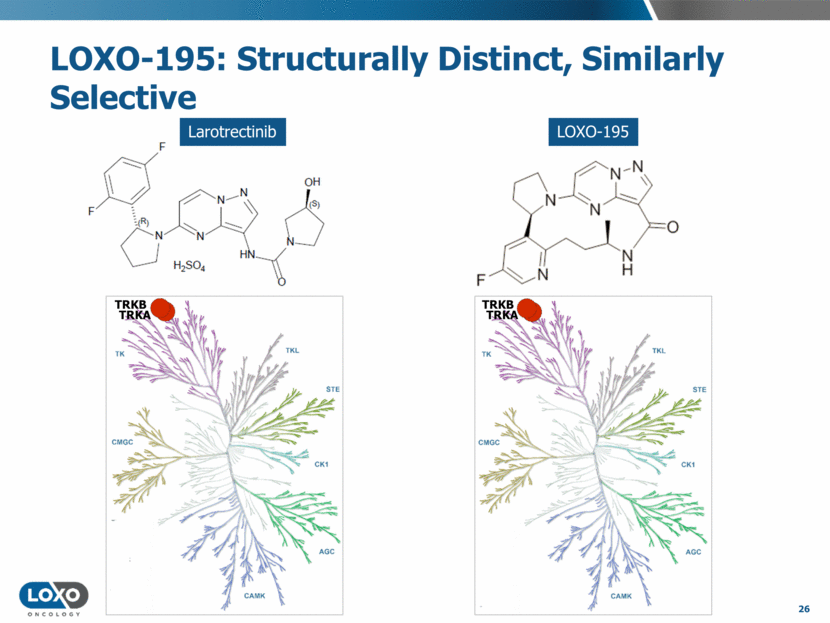
LOXO-195 is a potent and selective TRK inhibitor capable of addressing potential mechanisms of acquired resistance that may emerge in patients receiving larotrectinib (LOXO-101) or multikinase inhibitors with anti-TRK activity. LOXO-195 demonstrated potent inhibition of TRK fusions, including critical acquired resistance mutations, in enzyme and cellular assays, with minimal activity against other kinases. In diverse TRK fusion mouse models, LOXO-195 inhibited phospho-ERK and caused dramatic tumor growth inhibition, superior to first generation TRK inhibitors, without significant toxicity.
Tropomyosin-related kinase (TRK) is a receptor tyrosine kinase family of
neurotrophin receptors that are found in multiple tissues types. Three members of the TRK proto-oncogene family have been described: TrkA, TrkB, and TrkC, encoded by the NTRKI, NTRK2, and NTRK3 genes, respectively. The TRK receptor family is involved in neuronal development, including the growth and function of neuronal synapses, memory
development, and maintenance, and the protection of neurons after ischemia or other types of injury (Nakagawara, Cancer Lett. 169: 107-114, 2001).
TRK was originally identified from a colorectal cancer cell line as an oncogene fusion containing 5′ sequences from tropomyosin-3 (TPM3) gene and the kinase domain encoded by the 3′ region of the neurotrophic tyrosine kinase, receptor, type 1 gene (NTRKI) (Pulciani et al., Nature 300:539-542, 1982; Martin-Zanca et al., Nature 319:743-748, 1986). TRK gene fusions follow the well-established paradigm of other oncogenic fusions, such as those involving ALK and ROSl, which have been shown to drive the growth of tumors and can be successfully inhibited in the clinic by targeted drugs (Shaw et al., New Engl. J. Med. 371 : 1963-1971, 2014; Shaw et al., New Engl. J. Med. 370: 1189-1197, 2014). Oncogenic TRK fusions induce cancer cell proliferation and engage critical cancer-related downstream signaling pathways such as mitogen activated protein kinase (MAPK) and AKT (Vaishnavi et al., Cancer Discov. 5:25-34, 2015). Numerous oncogenic rearrangements involving
NTRK1 and its related TRK family members NTRK2 and NTRK3 have been described (Vaishnavi et al., Cancer Disc. 5:25-34, 2015; Vaishnavi et al., Nature Med. 19: 1469-1472, 2013). Although there are numerous different 5′ gene fusion partners identified, all share an in-frame, intact TRK kinase domain. A variety of different Trk inhibitors have been developed to treat cancer (see, e.g., U.S. Patent Application Publication No. 62/080,374,
International Application Publication Nos. WO 11/006074, WO 11/146336, WO 10/033941, and WO 10/048314, and U.S. Patent Nos. 8,933,084, 8,791, 123, 8,637,516, 8,513,263, 8,450,322, 7,615,383, 7,384,632, 6, 153,189, 6,027,927, 6,025,166, 5,910,574, 5,877,016, and 5,844,092).
LOXO-195 (TRK inhibitor)
LOXO-195 is a next-generation, selective TRK inhibitor capable of addressing potential mechanisms of acquired resistance that may emerge in patients receiving larotrectinib (LOXO-101) or multikinase inhibitors with anti-TRK activity.
Acquired resistance to targeted therapies has proven to be an important component of long-term cancer care and targeted therapy drug development. LOXO-195 was developed in anticipation of potential resistance to larotrectinib (LOXO-101), and in light of recent published literature regarding emerging mechanisms of resistance to TRK inhibition. With the LOXO-195 program, Loxo Oncology has an opportunity to clinically extend the duration of disease control for patients with TRK-driven cancers.
In May 2017, Loxo Oncology received clearance from the U.S. Food and Drug Administration for an Investigational New Drug (IND) application for LOXO-195. Loxo Oncology plans to initiate a multi-center Phase 1/2 study in mid-2017. The primary objective of the trial is to determine the maximum tolerated dose or recommended dose for further study. Key secondary objectives include measures of safety, pharmacokinetics, and anti-tumor activity (i.e. Objective Response Rate and Duration of Response, as determined by RECIST v1.1). The trial will include a dose escalation phase and dose expansion phase.

Data presented at the 2016 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics illustrated the potency, specificity, and favorable in vivo properties in animals of LOXO-195, our clinical candidate. Read the poster presented at AACR-NCI-EORTC here.
A research brief published in Cancer Discovery in June 2017 outlines the preclinical rationale for LOXO-195 and clinical proof-of-concept data from the first two patients treated. Read the publication here.
1H NMR PREDICT

13C NMR PREDICT
WO 2017075107
Can·cer, redefined
Lisa M. Jarvis
C&EN Global Enterp, 2017, 95 (27), pp 26–30
Publication Date (Web): July 3, 2017 (Article)
DOI: 10.1021/cen-09527-cover
http://pubs.acs.org/doi/full/10.1021/cen-09527-cover
C&EN, 2017, 95 (27), pp 26–30July 3, 2017

When Adrienne Skinner was diagnosed with ampullary cancer, a rare gastrointestinal tumor, in early 2013, it didn’t come as a complete surprise. For nearly a decade, she had known her genes were not in her favor. What she didn’t know was that her genes would also point the way to a cure. Skinner has Lynch syndrome, an inherited disorder caused by a defect in mismatch repair (MMR) genes, which encode for proteins that spot and fix mistakes occurring during DNA replication. People with Lynch syndrome have an up to 70% risk of developing colon cancer. Women with the disorder have similarly high chances of developing endometrial cancer at an early age. The first time Skinner heard about the syndrome was in late 2004, after her sister was diagnosed with colon, ovarian, and endometrial cancers, the telltale trifecta associated with Lynch syndrome. It turned out that Skinner, her sister, and their
- A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors
- Clinical Safety and Activity from a Phase 1 Study of LOXO-101, a Selective TRKA/B/C Inhibitor, in Solid Tumor Patients with NTRK Gene Fusions
- The Development of LOXO-195, a Second Generation TRK Kinase Inhibitor that Overcomes Acquired Resistance to First Generation Inhibitors in Patients with TRK-Fusion Cancers
- Acquired Resistance to the TRK Inhibitor Entrectinib in Colorectal Cancer
- What Hides Behind the MASC: Clinical Response and Acquired Resistance to Entrectinib After ETV6-NTRK3 Identification in a Mammary Analogue Secretory Carcinoma (MASC)
/////////LOXO 195, 2097002-61-2














