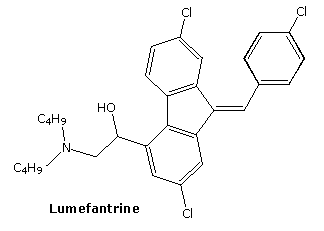
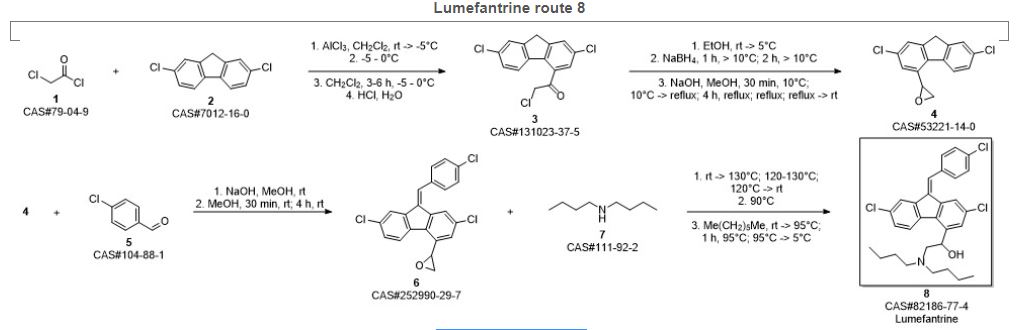
lumefantrine
- Molecular FormulaC30H32Cl3NO
- Average mass528.940 Da
UNIIF38R0JR742
CAS number82186-77-4
(±)-2-(Dibutylamino)-1-((9Z)-2,7-dichloro-9-(4-chlorobenzylidene)-9H-fluoren-4-yl)ethanol
(±)-2,7-Dichloro-9-((Z)-p-chlorobenzylidene)-α-((dibutylamino)methyl)fluorene-4-methanol
(9Z)-2,7-Dichloro-9-[(4-chlorophenyl)methylene]-α-[(dibutylamino)methyl]-9H-fluorene-4-methanol
2-(dibutylamino)-1-[(9Z)-2,7-dichloro-9-[(4-chlorophenyl)methylidene]-9H-fluoren-4-yl]ethan-1-ol
| (±)-2,7-Dichloro-9-((Z)-p-chlorobenzylidene)-α-((dibutylamino)methyl)fluorene-4-methanol |
| 2-Dibutylamino-1-[2,7-dichloro-9-(4-chloro-benzylidene)-9H-fluoren-4-yl]-ethanol |
| 2-Dibutylamino-1-{2,7-dichloro-9-[1-(4-chloro-phenyl)-meth-(Z)-ylidene]-9H-fluoren-4-yl}-ethanol |
| Benflumetol |
| dl-Benflumelol |
UNII F38R0JR742
CAS number 82186-77-4
Weight Average: 528.94
Monoisotopic: 527.154947772
Chemical Formula C30H32Cl3NO
Lumefantrine, also known as HSDB 7210, is an antimalarial agent used to treat acute uncomplicated malaria. It is administered in combination with artemether for improved efficacy. This combination therapy exerts its effects against the erythrocytic stages of Plasmodium spp. and may be used to treat infections caused by P. falciparum and unidentified Plasmodium species, including infections acquired in chloroquine-resistant areas.
Lumefantrine (or benflumetol) is an antimalarial drug. It is only used in combination with artemether. The term “co-artemether” is sometimes used to describe this combination.[1] Lumefantrine has a much longer half-life compared to artemether and so is therefore thought to clear any residual parasites that remain after combination treatment.[2]
Lumefantrine, along with pyronaridine and naphtoquine, were synthesized in course of the Project 523 antimalaria drug research initiated in 1967; these compounds are all used in combination antimalaria therapies.[3][4][5]

Lumefantrine is an antimalarial drug chemically known as 2-(dibutylamino)-1-[(9Z)-2, 7-dichloro-9-(4- chlorobenzylidene)-9H-floren-4-yl] ethanol, which is used in the prevention and treatment of Malaria in worm blooded animals. Lumefantrine is using the combination of β-Artemether in the treatment of Malaria
SYN
Synthetic Reference
Beutler, Ulrich; Fuenfschilling, Peter C.; Steinkemper, Andreas. An Improved Manufacturing Process for the Antimalaria Drug Coartem. Part II. Organic Process Research & Development. Volume 11. Issue 3. Pages 341-345. 2007.
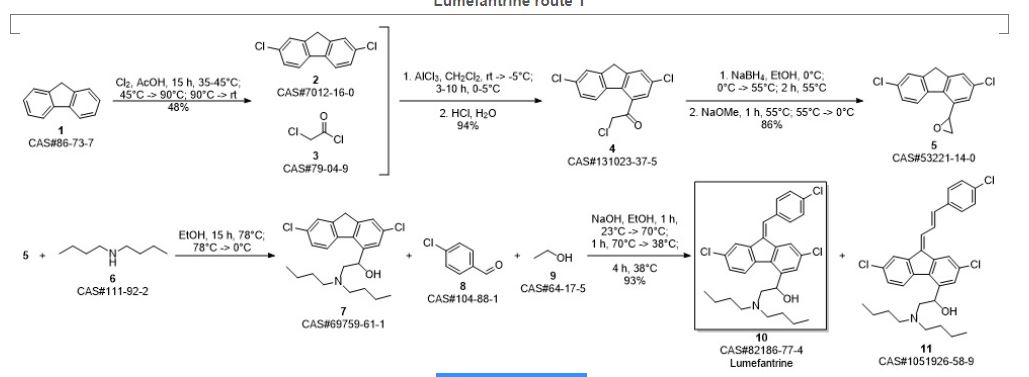
SYN 2
Synthetic Reference
Rao, Dharmaraj Ramachandra; Kankan, Rajendra Narayanrao; Phull, Manjinder Singh. Process for preparation of lumefantrine as antimalarial agent with improved method. Assignee Cipla Co., Ltd., India. CN 1865227. (2006).
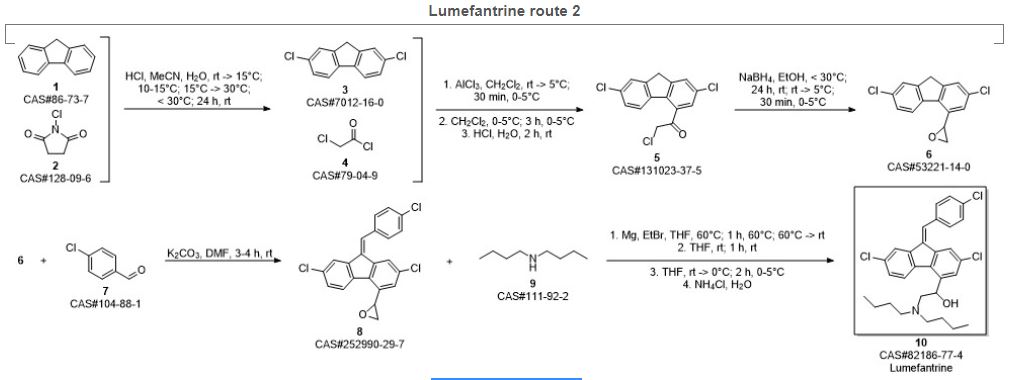
SYN 3
Synthetic Reference
Sethi, Madhuresh Kumar; Gonuguntla, Anantavena Rani; Arikatla, Siva Lakshmi Devi; Mulukutla, Suryanarayana; Yerramalla, Rajakrishna; Bontalakoti, Jaganmohanarao; Vemula, Lakshminarayana; Thirunavukarasu, Jayaprakash. Synthesis and characterization of novel related substances of Lumefantrine, an anti-malarial drug. Pharma Chemica. Volume 8. Issue 3. Pages 91-100. 2016.
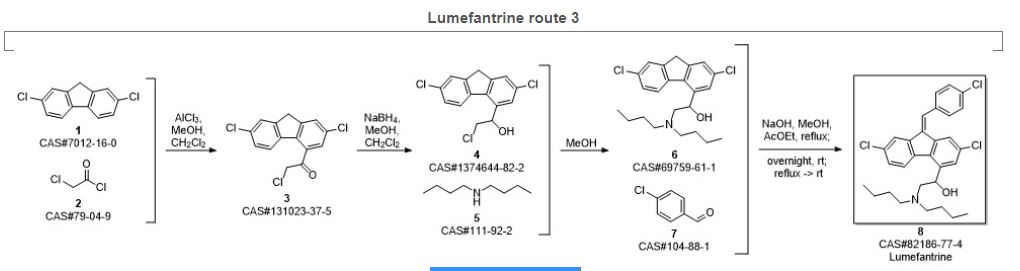
SYN4
Synthetic Reference
Mathur, Prafull; Mathur, Suvigya; Vishwanath, Kannan; Mishra, Anand Kumar. Preparation of lumefantrine. Assignee Aanjaneya Lifecare Limited, India. IN 2013MU00611. (2015).
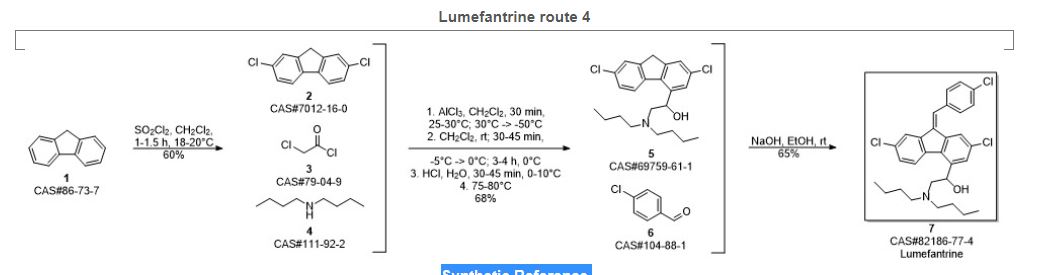
SYN 5
Synthetic Reference
Wu, Guang-liang; Dai, Ying-jie; Kang, Cong-min; Zi, Yan. A new synthetic technology of anti-malarial drug lumefantrine. Zhongguo Xinyao Zazhi. Volume 21. Issue 24. Pages 2944-2947. 2012.
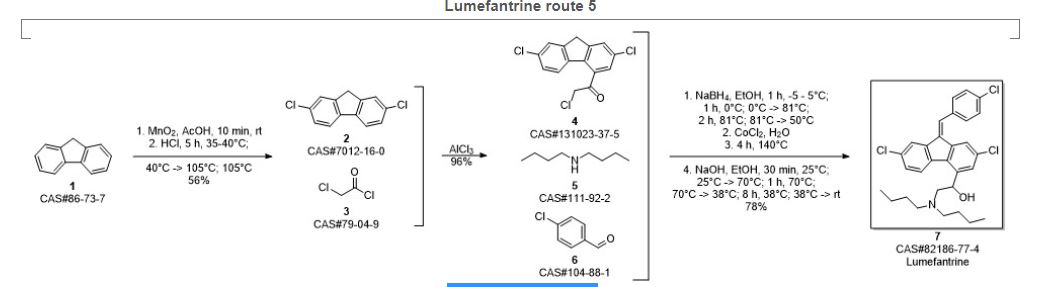
SYN 6
Synthetic Reference
Krishna, Bettadapura Gundappa; Verma, Sudhakar; Krishna, Sujatha; Naik, Gajanan; Arulmoli, Thangavel. A process for preparation of lumefantrine. Assignee SeQuent Scientific Limited, India. IN 2012CH00470. (2012).
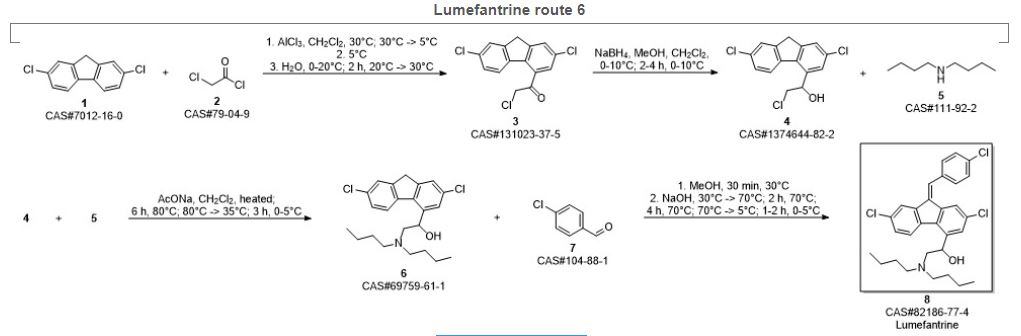
SYN 7
Synthetic Reference
Bansi, Lal; Genbhau, Gund Vitthal; Prabhakar, Bapat Chintamani; Popat, Bochiya Pravin; Banshi, Punde Dnyanadeo; Venkata, Reddy Prabhakar Gorla. Improved one pot process for the synthesis of lumefantrine. Assignee Calyx Chemicals and Pharmaceuticals Ltd., India. IN 2009MU01437. (2010).
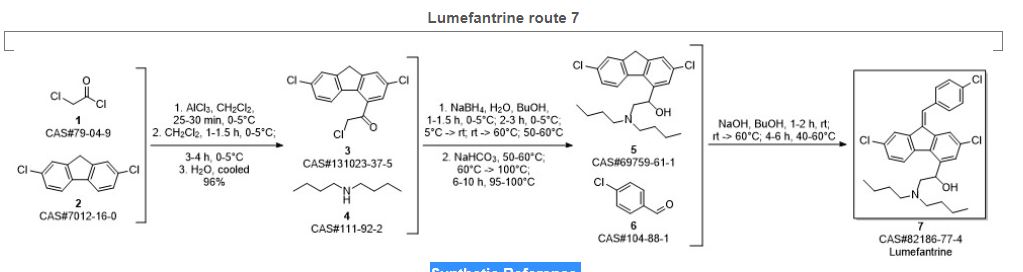
SYN 8
Synthetic Reference
Shailesh, Singh; Dhaval, Vashi; Vinod, Gaikwad; Sanjay, Chowkekar; Sanjay, Bute. Process for the preparation of lumefantrine. Assignee Ajanta Pharma Ltd., India. IN 2008MU01677. (2010).
SYN 9
Synthetic Reference
Rawalnath, Sakhardande Rajiv; Kanji, Khatri Navin; Nilkanth, Firake Pandharinath; Vasant, Panchal Rajesh; Nagesh, Babrekar Chandan; Madhukar, Mohite Dhanaji. Preparation of lumefantrine. Assignee Saxena, Alok, India. IN 2006MU00260. (2007).
PATENT
https://patents.google.com/patent/CN107501316A/en
Embodiment 1:The synthesis of (the chloro- 2- of 2- (the chloro- 9H- fluorenes -4- bases of 2,7- bis-) ethyoxyl) trimethyl silane
2,7- dichloro fluorenes -4- oxirane (100g, 0.368mol), zinc oxide are sequentially added in 1000ml there-necked flasks (2.94g, 0.036mol) and 500ml dichloromethane, TMSCl (43g, 0.4mol) are added drop-wise in above-mentioned reaction solution, room temperature reaction 2h.Filtering, a small amount of dichloromethane washing of filter cake, filtrate washing, organic phase anhydrous sodium sulfate drying, filtering, filtrate are rotated to analysis After going out solid, stop revolving, stand and separate out a large amount of yellow solids, filtering, drain to obtain (the chloro- 2- of 2- (2,7- bis- chloro- 9H- fluorenes- 4- yls) ethyoxyl) trimethyl silane yellow solid 102g, yield 73%.δ(1HNMR,CDCl3):7.78-7.80(m,1H), 7.56(s,1H),7.48(s,1H),7.41(s,1H),7.32-7.35(m,1H),5.64-5.67(m,1H),4.03-4.11(m, 2H),3.84(m,2H),0.63(s,9H),ppm。
Embodiment 2:N- butyl-N- (1- (the chloro- 9H- fluorenes -4- bases of 2,7- bis-) -2- (trimethylsiloxy group) ethyl) butyl – The synthesis of 1- amine:
(the chloro- 2- of 2- (the chloro- 9H- fluorenes -4- bases of 2,7- bis-) ethyoxyl) trimethyl silicane is sequentially added in 1000ml there-necked flasks Alkane (100g, 0.26mol), di-n-butylamine (67g, 0.52mol), potassium carbonate (71.65g, 0.52mol) and 800ml acetonitriles.Put It is changed to nitrogen system, back flow reaction 16h.After being down to room temperature, filtering, a small amount of acetonitrile of filter cake washs, and filtrate is evaporated to obtain fourth containing N- The crude yellow oil of base-N- (1- (the chloro- 9H- fluorenes -4- bases of 2,7- bis-) -2- (trimethylsiloxy group) ethyl) butyl -1- amine. Crude product is directly used in be synthesized in next step, without purifying.MS:479(MH+)
Embodiment 3:The synthesis of 2- dibutyl aminos -2- [the chloro- 9H- fluorenes -4- bases of 2,7- bis-] ethanol
By-the N- of butyl containing N- obtained in the previous step (1- (the chloro- 9H- fluorenes -4- bases of 2,7- bis-) -2- (trimethylsiloxy group) second Base) crude yellow oil of butyl -1- amine is dissolved in 500ml tetrahydrofurans, concentrated hydrochloric acid (1ml), nitrogen protection, heating is added dropwise To 50 DEG C of reactions, TLC tracing detections to raw material have reacted completely, cool, and separate out solid, filtering, the washing of filter cake tetrahydrofuran, take out Dry, filtrate is abandoned, and filter cake is transferred in beaker, adds 200ml dichloromethane, and saturated aqueous sodium carbonate is adjusted pH=8, separated Machine phase, water layer are extracted once with 200ml dichloromethane again, merge organic phase, are washed, and are dried, are concentrated to give yellow oil 68g, two step yields 64%.δ(1HNMR,CDCl3):7.80(s,1H),7.56(s,1H),7.38(m,2H),7.25(s,1H), 4.7(brs,1H),4.03-4.11(m,2H),3.99(m,1H),3.56(m,2H),2.42-2.85(m,4H),1.44-1.48 (m,4H),1.23-1.28(m,4H),0.86-0.91(m,6H).ppm。
Embodiment 4:(RS, Z) -2- dibutyl aminos -2- [bis- chloro- 9- of 2,7- (4- chlorobenzenes methylene) -9H- fluorenes -4- bases] second The synthesis of alcohol
2- dibutyl aminos -2- [the chloro- 9H- fluorenes -4- bases of 2,7- bis-] ethanol (68g, 0.167mol) is dissolved in 200ml ethanol In, 4-chloro-benzaldehyde (28.2g, 0.2mol) and sodium hydroxide (2.68g, 0.067mol) are added, nitrogen protection, is heated to reflux anti- Answer 1h.Room temperature is down to, watery hydrochloric acid, which is added dropwise, makes product be filtered into salting out, and the washing of filter cake ethanol, drains to obtain yellow solid, shifts Into beaker, 200ml dichloromethane is added, adds saturated aqueous sodium carbonate to dissociate, be layered, aqueous phase uses 200ml dichloromethane again Extraction once, merges organic phase, washing, and anhydrous sodium sulfate drying 6h is filtered, and filtrate is concentrated to give (RS, Z) -2- dibutyl aminos -2- [2,7- bis- chloro- 9- (4- chlorobenzenes methylene) -9H- fluorenes -4- bases] ethanol be yellow foam shape solid 74g, yield 83%, 0-5 DEG C Preserve.δ(1HNMR,CDCl3):7.75(brs,1H),7.66(s,1H),7.55(s,1H),7.44-7.48(m,5H),7.28- 7.29(m,1H),7.30-7.31(m,1H),4.7(brs,2H),3.88-3.89(m,2H),2.71-2.72(m,2H),2.60- 2.63(m,2H),1.44-1.48(m,4H),1.23-1.31(m,4H),0.86-0.90(m,6H).ppm。
PATENT
Process for high purity lumefantrine Dr KrishnaSarma Pathy,Ramesh.D,P atchutaramaiah,Ch Sivasubramanyam Abstract. The present invention relates to Process for the preparation of lumefantrine Lumefantrine is a dichlorobenzylidine derivative effective for the treatment of various types of malaria. Chemically lumefantrine is 2-Dibutylamino-1-[ 2, 7-dichloro-9-(4- chlorobenzylidene)-9H-fluoren-4-yl]-Ethanol (racemate) The antimalarial agent is active against multi-drug resistant strains of Plasmodium falciparum. In combination with artemether, the drug is also used for the treatment of uncomplicated falciparum malaria. It has primary action as blood schizontocidal and secondary action as inhibition of nucleic acid and protein synthesis within the malarial parasite thus having a longer duration of anti malarial action. Thus, today lumefantrine is a drug of choice in antimalarial treatment against P. faliciparum. Therefore, development of an appropriate analytical procedure for the quantitative analysis of lumefantrine is of considerable importance to pharmaceutical industry. The spectroscopic techniques were used to confirm the identity of lumefantrine. The IR spectra, showed strong absorption band at 3404.67 cm-1(OH), 2953.28 cm-1(aliphatic and aromatic CH), 1757.31 cm-1(-C=C-), 933 cm-1(alkanes) and 696.37-373.22 cm-1 (Cl). Thus, IR spectra confirmed the presence of these functional groups in the structure of lumefantrine. The mass spectrum showed a sharp molecular ion peak at 528.0 m/z in Q1 MS (m/z, parent ion) parameter at negative polarity confirming the molecular weight of lumefantrine. The NMR spectra observed triplet at 0.943-0.989 (methyl protons of alkyl chain); a multiplet at 1.372-1.498 (methylene protons of alkyl chains); a multiplet at 2.449-2.909 (methylene protons of alkyl chain); broad singlet at 4.573 (OH proton); and multiplet at 7.314-7.733 (aromatic proton), thus confirming identity of lumefantrine. Summary of the process Stage1 Cl Cl 2,7-dichloro-9H-fluorene ClCl O Cl 2-chloro-1-(2,7-dichloro-9H-fluoren-3-yl)ethanone AlCl3 Chloroacetyl chloride In a 1 liter 3-necked flask, equipped with stirrer, thermometer and reflux condenser450.0 ml MDC cool to 0C.Start addition of 59.4 gm chloro acetyl chloride at 0-5C and stir for 15 min.Start addition of aluminium chloride at 0-5C. Stir for 30.0 min at same temp.Start addition of 2,7-dichloro fluorene soln. preprepared in 300.0 ml MDC d 150.0 ml saturated brine for washing and stir for 15-20 min. at 50-55 C.Allow to settle the layers at 50- 55C for 30.0 min.Separate the ORGANIC LAYER. at 50-55C.Again repeat Abovestep. for one time.Collect ORGANIC LAYER. and distill out dibutyl amine under vac. At 60- 90C.Disconnect vac. And cool reaction mass to 60C add 400.0 ml methanol and reflux to 30.0 min. After maintaining the reaction mass cool reaction mass to 50C.Seed the reaction mass by adding 500 mg stage 2 material at . 50C.Cool reaction mass to 30- 35C.Stir reaction mass further at same temp. fot 2 hrs.Filter reaction mass at 30- 35C.Suck dry and wash with 50.0 ml methanol at 30-35C.Suck dry and unload. Dry at 50-55C. Wt. of wet cake : 139.0 gm Wt. of dry material : 123.0 gm . . . a b c . . . a b c M.P: 72-76C Stage3: ClCl N OH + Cl COH ClCl N OH Cl 2,7-dichloro-alpha-[(dibutyl amino) p-chloro benzaldehyde methyl]-9H-fluoren-4-yl]oxirane Mol. Wt. 406 Lumefantrine Mol. Wt. 140.5Mol. Wt. 528.5 Under Nitrogen In a 2 liter 3-necked flask, equipped with stirrer, thermometer and reflux.Charge 840.0 ml ethanol at 30-35C.Chrge 120.0gm stage2 material at 30-35C.Charge 27.96 gm sodium methoxide in 30 min. at 30C exothermobserved temp. rise to 45C.Charge 41.52 gm p- Chloro benzaldehyde .Maintain reaction mass temp. 30-35C for 24 hrs. Check TLC a : Stage 2 b : Co spot c : reaction mass Mobile phase Hexane : Ethyl acetate 9 : 1 If TLC complies 1) 2) 3) Filter reaction mass at 30-35C. Suck dry and wash with 100.0 ml methanol. Suck dry and unload wet cake. Dry material at 50-55 C . . . a bc Wt. of wet cake : 184.0 gm Wt. Of dry material : 141.0 gm M.P : 122-126C Purification : In a 1 liter 3-necked flask, equipped with stirrer, thermometer and reflux .Charge 700.0 ml ethyl acetate at 30-35C.Chrge crude 141.0 gm material at 30-35C.Heat reaction mass to refluxand main for 30.0 min. to dissolve the material.Filter reaction mass at 75-80C.Collect filtrate cool to reaction mass to 0- 5C.Maintain reaction mass at same temp. for 30.0 min.Filter the reaction mass at 0- 5C.Suck dry and wash with 100.0 ml ethyl acetate at 0-5C.Suck dry and unload. Dry material at 50-55 C Wt. of wet cake : 100.0 gm Wt. Of dry material : 70-80 gm
(19) (PDF) Process for high purity lumefantrine. Available from:
https://www.researchgate.net/publication/221933417_Process_for_high_purity_lumefantrine [accessed Feb 06 2022].
M.P: 128-132C RESULTS:= Lumefantrine 99.15% (by HPLC) 128 – 130 Purity Melting Pointo C Liquid chromatography HPLC-UV investigation of the impurity profiles was performed on a HPLC-PDA apparatus consisting of a Waters Alliance 2695 separation module and a Waters 2998 photodiode array detector with Empower 2 software for data acquisition . For PDA detection, the UV spectrum was recorded at 190-400 nm. Quantification was performed at 266 nm. The positive ion ESI and the collision-induced dissociation (CID) mass spectra were obtained from the LC-UV/MS apparatus consisting of a Spectra System SN4000 interface, a Spectra System SCM1000 degasser, a Spectra System P1000XR pump, a Spectra System AS3000 autosampler, and a Finnigan LCQ Classic ion trap mass spectrometer in positive ion mode mass to charge range m/z 100 to m/z 2000 at unit resolution and with a peak width of 0.25 daltons/z, equipped with a Waters 2487 dual wavelength UV detector and Xcalibur 2.0 software (Thermo) for data acquisition. ESI was conducted using a needle voltage of 4.5 kV. Nitrogen was used as sheath and auxiliary gas with the heated capillary set at 250C. UV-detection was used for quantification (at 266 nm), while ESI-ion trap MS detection was used for identification. LC determination of impurities in lumefantrine samples was performed using a Purospher STAR RP-18 endcapped (150 4.6 mm, 5 m particle size) column (Merck, Darmstadt, Germany) with guard column at 30C under isocratic conditions with a mobile phase consisting of ammonium acetate (pH 4.9; 0.1 M) and acetonitrile (10:90, v/v). The flow rate was set at 2.0 mL/min (minimal run time: 30 min.). The injection volume was 10 l. Under these conditions, lumefantrine elutes at approximately 22 min. System suitability tests (SSTs) were established as the plate number on lumefantrine (N 8.2 103) and the oxidative stress degradation product (N 2.4 103), the signal-to-noise ratio of 0.5% l.c. lumefantrine solution (S/N 30), the peak area ratio of the 0.5% l.c. versus 100% l.c. (between 0.4 and 0.6) and the relative position of the in-situ prepared N-oxide by H2O2 treatment (RRTbetween 0.12 and 0.22). The LC method was validated for the determination of lumefantrine and its related impurities. The selectivity of the developed chromatographic method was established by the separation of lumefantrine and its impurities. A correlation coefficient (r2) of 0.9998 for lumefantrine (0.0006 to 0.01 mg/ml) and 1.0 for impurity A and DBK (0.001 to 0.1 mg/ml) demonstrated that the HPLC method is linear in the lower range. LOD/LOQ values for lumefantrine, DBK and impurity A were calculated (S/N = 3 resp. 10): 0.004 mg/mland 0.026 mg/ml for lumefantrine (0.004% respectively 0.026% l.c.), 0.011 mg/ml and 0.040 mg/ml for DBK (0.012% respectively 0.042% l.c.) and 0.110 mg/ml and 0.393 mg/ml for impurity A (0.115% respectively 0.409% l.c.). The analytical stability of lumefantrine, impurity A and DBK was confirmed over a storage period of 24 hours at 5C, i.e. the sample compartment temperature. Accuracy and precision were evaluated by repeated analysis (n = 6), with 102.6% l.c. recovery and 2.1%, respectively 2.86%, for repeatability, respectively intermediate precision. Structural information for the observed and/or reported lumefantrine related impurities # Compound [formula, mono-isotopic mass]Structure Origin # Compound [formula, mono-isotopic mass]Structure Origin Alkaline stress Oxidative stress Oxidative stress Metabolite 1Desbenzylketo N-oxide [C23H27NO3Cl2, MW 435.14] 2 Lumefantrine (mono-)desbutyl derivative [C26H24NOCl3, MW 473.09] 3Lumefantrine N-oxide [C30H32NO2Cl3, MW 543.15] Oxidative stress Degradation 4 2,7-dichloro-4-[2-(di-n-butylamino)-1-hydroxyethyl]- 9H-fluoren-9-one; Desbenzylketo derivative (DBK) [C23H27NO2Cl2, MW 419.14] Oxidative stress Acidic stress Degradation 5 2-(di-n-butylamino)-1-[2,7-dichloro-9H-fluoren-4- yl]ethanol; Desbenzyl derivative [C23H29NOCl2,405.16] Synthesis 6 Synthesis impurity found in lumefantrine API; Lumefantrine oxide [C30H32NO2Cl3, MW 543.14] Synthesis 7 Synthesis impurity found in lumefantrine API; Lumefantrine oxide [C30H32NO2Cl3, MW 543.14] Synthesis 8 (RS,Z)-2-(Dibutylamino)-2-(2,7-dichloro-9-(4- chloro-benzylidene)-9H-fluoren-4-yl)ethanol (isomeric compound); Impurity A (Ph. Int./USP Salmous) [C30H32NOCl3, MW 527.15] Synthesis 9 Synthesis
(19) (PDF) Process for high purity lumefantrine. Available from:
https://www.researchgate.net/publication/221933417_Process_for_high_purity_lumefantrine [accessed Feb 06 2022].
mpurity found in lumefantrine API; Lumefantrine oxide [C30H32NO2Cl3, MW 543.14] Synthesis 10 (1S,3R,5R)-1,3-bis((EZ)-2,7-Dichloro-9-(4- chlorobenzyl-idene)-9H-fluoren-4-yl)-2,6- dioxabicyclo[3.1.0]hexane; Impurity BA(USP Salmous) [C44H24Cl6O2, 797.39] 2-((EZ)-2,6-Dichloro-9- (4-chlorobenzylidene)-9H- fluoren-4-yl)-3′-((EZ)-2,7-dichloro-9-(4- chlorobenzylidene)-9H-fluoren-4-yl)-2,2′-bioxirane; Impurity BB (USP Salmous) [C44H24Cl6O2, 797.39] Percentage maximum actual levels of lumefantrine related impuritiesobserved(1) Synthesis 11 Synthesis # CompoundAPIFPP Unstressed Stressed Release Accelerated Stressed 1.39 0.560.11 21.32 1 Desbenzylketo N-oxide 2 Monodesbutyl derivative 3 Lumefantrine N-oxide 4 Desbenzylketo derivative 5 Desbenzyl derivative 6Lumefantrine oxide (RRT ~ 0.49) 7Lumefantrine oxide (RRT ~ 0.52) 8 Impurity A 9Lumefantrine oxide (RRT ~ 0.59) (1) RT: reporting threshold = 0.10% 0.60 0.12 0.34 0.86 4.26 HPLC characteristics of lumefantrine related impurities # CompoundRT(1)RRT(2) Most abundant m/z observed 436.14 474.00 544.08 420.13 406.09 544.12 544.12 528.10 544.12 528.10 1 Desbenzylketo N-oxide 2 Monodesbutyl derivative 3.25 3 Lumefantrine N-oxide 4 Desbenzylketo derivative 7.41 5 Desbenzyl derivative 6 Lumefantrine oxide 7 Lumefantrine oxide 8 Impurity A 9 Lumefantrine oxide L Lumefantrine (1) Retention time (min.) (2) Relative retention time reference 1.790.08 0.15 0.17 0.33 0.34 0.49 0.52 0.58 0.59 1.00 3.96 7.69 10.96 11.45 12.70 12.97 22.28 1. De Spiegeleer B, Vergote V, Pezeshki A, Peremans K, Burvenich C. Impurity profiling quality control testing of synthetic peptides using liquid chromatography-photodiode array-fluorescence and liquid chromatography-electrospray ionization-mass spectrometry: The obestatin case. Anal Biochem. 2008;376:229234. doi: 10.1016/j.ab.2008.02.014.[ 2. Nicolas EC, Scholz TH. Active drug substance impurity profiling – Part I. LC/UV diode array spectral matching. J Pharm Biomed Anal. 1998;16:813824. doi: 10.1016/S0731-7085(97)00131- 3Roy J. Pharmaceutical impurities – A mini review. AAPS PharmSciTech. 2002;3:article 6. doi: 10.1208/pt030206. ICH guidelines – International Conference on Harmonization, Q3A(R2) Impurities in new drug substances CPMP/ICH/2737/99. (October 2006) http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC50000 2675.pdf [Accessed on 5 November 2010 at 11:06] 3. Authorized Lumefantrine USP Salmous Standard (Februari 2009) http://www.usp.org/pdf/EN/nonUSStandards/lumefantrine.pdf [Accessed on 24 October 2010 at 17:14] 4. Lumefantrine: Document QAS/06.186/FINAL (WHO Ph. Int. – July 2008) http://www.who.int/medicines/publications/pharmacopoeia/Lumef_monoFINALQAS06_186_July 08.pdf [Accessed on 24 October 2010 at 17:37] 5. Lee H. Pharmaceutical applications of liquid chromatography coupled with mass spectrometry (LC/MS) J Liq Chromatogr Relat Technol. 2005;28:11611202. doi: 10.1081/JLC-200053022.] 6. Cesar ID, Nogueira FHA, Pianetti GA. Simultaneous determination of artemether and lumefantrine in fixed dose combination tablets by HPLC with UV detection. J Pharm Biomed Anal. 2008;48:951954. doi: 10.1016/j.jpba.2008.05.022 7. Cesar ID, Nogueira FHA, Pianetti GA. Comparison of HPLC, UV spectrophotometry and potentiometric titration methods for the determination of lumefantrine in pharmaceutical products. J Pharm Biomed Anal. 2008;48:223226. doi: 10.1016/j.jpba.2008.05.006. 8. Patil KR, Rane VP, Sangshetti JN, Shinde DB. A Stability-Indicating LC Method for Lumefantrine. Chromatographia. 2009;69:375379. doi: 10.1365/s10337-008-0894-x. [Cross Ref] 9. Munjal V, Paliwal N, Chaursia BK, Varshney B, Ahmed T, Paliwal J. LC-tandem mass spectrometry method for quantification of lumefantrine in human plasma and its application to bioequivalence study. Chromatographia. 2010;71:505510. doi: 10.1365/s10337-009-1446-8. [Cross Ref] 10. ICH guidelines – International Conference on Harmonization, Q1A(R2) Stability testing of new drug substances and products CPMP/ICH/2736/99. (August 2003) http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC5
(19) (PDF) Process for high purity lumefantrine. Available from:
https://www.researchgate.net/publication/221933417_Process_for_high_purity_lumefantrine [accessed Feb 06 2022].
PATENT
PAPER
Tropical Journal of Pharmaceutical Research October 2013; 12 (5): 791-798 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org
CLIP
NMR
https://www.researchgate.net/publication/338694609_Structural_Characterization_and_Thermal_Properties_of_the_Anti-malarial_Drug_Lumefantrine/figures?lo=1

1H NMR

COSY

NOESY

HSQC

DEPT

13C NMR

DSC
CLIP
http://derpharmachemica.com/vol8-iss3/DPC-2016-8-3-91-100.pdf
REFERENCES
[1] Ulrich Beutler, C Peter.; Fuenfschilling.; and Andreas, Steinkemper.; Novartis Pharma AG; Chemical and Analytical Development: CH-4002 Basel, Switzerland, Organic Process Research & Development 2007, 11, 341- 345.
[2] Boehm, M. Fuenfschilling.; Krieger, P. C.; Kuesters, E. M.; Struber, F.; Org. Process Res. DeV. 2007, 11, 336- 340.
[3] (a) Rao, D. R.; Kankan, R. N.; Phull, M. S.; Patent Application CN 1009-3724 20060424, 2005. (b) Deng, R.; Zhong, J.; Zhao, D.; Wang, J.; Yaoxue, X. 2000, 35 (1), 22. (c) Allmendinger, Th.; Wernsdorfer, W. H. PCT WO 99/67197.
[4] Perrumattam, J.; Shao, Ch.; Confer, W. L. Synthesis 1994, 1181.
[5] Fuenfschilling, P. C.; Hoehn P.; Mutz J.-P. Organic Process Res. Dev. 2007, 11, 13.
[6] Di Nunno, L.; Scilimati, A. Tetrahedron 1988, 44, 3639.
[7] Pharmacopeial Forum, Vol. 36(2) [Mar.-Apr. 2010]
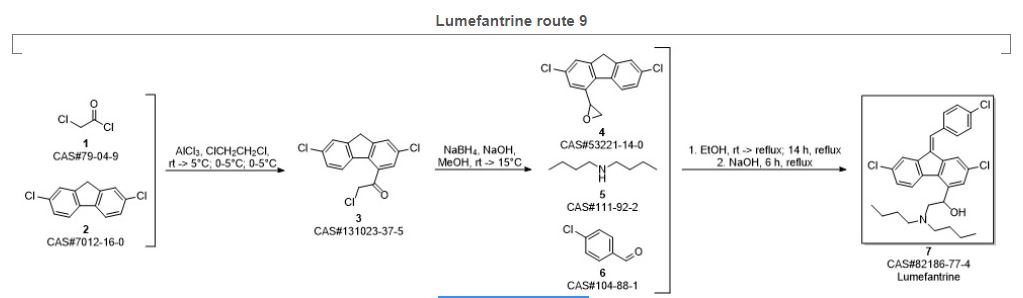
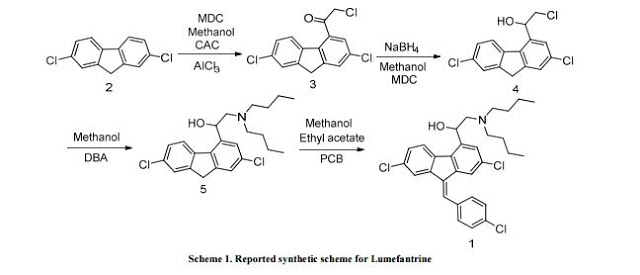
Preparation of 2-(dibutylamino)-1-[(9Z)-2, 7-dichloro-9-(4-chlorobenzylidene)-9H-floren-4-yl] ethanol (Lumefantrine) 1.
To a stirred solution of NaOH (1.97 g 0.0492 mol) in methanol (100 ml) there was added 1-(2, 7- dichloro-9 H-fluren-4-yl)-2-(dibutyl amino) ethanol (10 g, 0.0246 mol) and para chloro benzaldehyde (5.24 g 0.0372). The suspension obtained was stirred at reflux temperature till the absence of starting material by TLC. After confirming the product formation reaction mixture was cooled to room temperature and further stirred at same temperature for overnight. The precipitated solids were filtered and washed with methanol and dried under vacuum at 50°C to get desired compound. (Purity by HPLC: 99%).
IR (cm-1): 3408, 3092, 2953, 2928, 2870, 2840, 1634, 1589, 1487, 1465, 1443, 1400, 1365, 1308, 1268, 1241, 1207, 1173, 1156, 1085, 1071, 1014, 980, 933, 874, 839, 815, 806, 770;
1H NMR (CDCl3, δ ppm): 7.75 (d, 1H, CH, J 1.5 Hz), 7.68 (d, 1H, CH, J 1.5 Hz), 7.60-7.63 (m, 1H, CH), 7.32-7.35 (dd, 1H, CH, J 1.7,8.3 Hz), 7.45-7.50 (m, 1H, CH), 5.35-5.39 (dd, 1H, CH, J 3.0,9.9 Hz), 2.41-2.74 (m, 1H, CH2Ha), 2.86-2.92 (m, 1H, CH2Hb), 2.41-2.74 (m, 4H, CH2), 1.25-1.56 (m, 8H, CH2), 0.97 (t, 1H, CH, J 7.2 Hz), 7.60-7.63 (m, 1H, CH), 7.45-7.50 (m, 4H, CH), 4.54 (broad, 1H, OH),
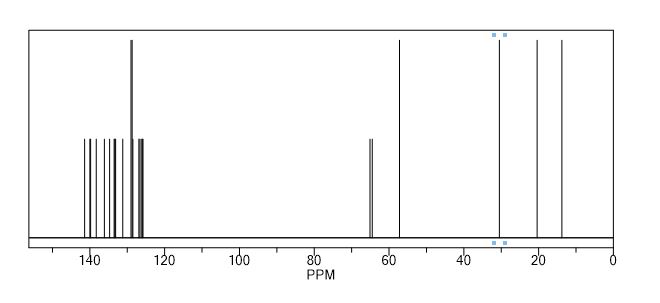
13C NMR (CDCl3, δ ppm): 138.2, 141.5, 120.6, 133.2, 126.3, 135.0, 135.0, 136.4, 123.9, 128.3, 132.8, 123.0, 139.8, 65.5, 60.0, 53.5, 29.1, 20.6, 14.0, 127.6, 134.7, 130.5, 129.1, 133.2;
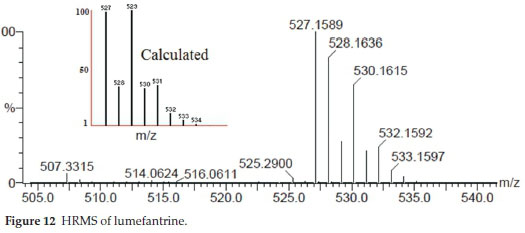
MS: m/z: 528 [M+H]+ ; Analysis calcd. for C30H32Cl3NO: C, 68.12; H, 6.10; N, 2.65% Found: C, 68.38; H, 6.14; N, 2.63 %.
CLIP

One-dimensional 1H NMR spectrum of B) a lumefantrine standard,
A CLIP
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532012000100010&lng=en&nrm=iso




CLIP
https://www.ncbi.nlm.nih.gov › NCBI › Literature › PubMed Central (PMC)
[2–4] Thus, today lumefantrine is a drug of choice in antimalarial treatment against P. …. The NMRspectra observed triplet at 0.943-0.989 (methyl protons of alkyl …
The spectroscopic techniques were used to confirm the identity of lumefantrine. The IR spectra, showed strong absorption band at 3404.67 cm-1 (OH), 2953.28 cm-1 (aliphatic and aromatic CH), 1757.31 cm-1 (-C=C-), 933 cm-1 (alkanes) and 696.37-373.22 cm-1 (Cl). Thus, IR spectra confirmed the presence of these functional groups in the structure of lumefantrine.
The mass spectrum showed a sharp molecular ion peak at 528.0 m/z in Q1 MS (m/z, parent ion) parameter at negative polarity confirming the molecular weight of lumefantrine.
The NMR spectra observed triplet at 0.943-0.989 (methyl protons of alkyl chain); a multiplet at 1.372-1.498 (methylene protons of alkyl chains); a multiplet at 2.449-2.909 (methylene protons of alkyl chain); broad singlet at 4.573 (OH proton); and multiplet at 7.314-7.733 (aromatic proton), thus confirming identity of lumefantrine.
IH NMR PREDICT
13C NMR PREDICT
References
- Jump up^ Toovey S, Jamieson A, Nettleton G (2003). “Successful co-artemether (artemether-lumefantrine) clearance of falciparum malaria in a patient with severe cholera in Mozambique”. Travel medicine and infectious disease. 1 (3): 177–9. doi:10.1016/j.tmaid.2003.09.002. PMID 17291911.
- Jump up^ White, Nicholas J.; van Vugt, Michele; Ezzet, Farkad (1999). “Clinical Pharmacokinetics and Pharmacodynamics of Artemether-Lumefantrine”. Clinical Pharmacokinetics. 37 (2): 105–125. doi:10.2165/00003088-199937020-00002. ISSN 0312-5963.
- Jump up^ Cui, Liwang; Su, Xin-zhuan (2009). “Discovery, mechanisms of action and combination therapy of artemisinin”. Expert Review of Anti-infective Therapy. 7 (8): 999–1013. doi:10.1586/eri.09.68. PMC 2778258
 . PMID 19803708.
. PMID 19803708.
- Jump up^ http://aac.asm.org/content/56/5/2465.full
- Jump up^ Laman, M; Moore, BR; Benjamin, JM; Yadi, G; Bona, C; Warrel, J; Kattenberg, JH; Koleala, T; Manning, L; Kasian, B; Robinson, LJ; Sambale, N; Lorry, L; Karl, S; Davis, WA; Rosanas-Urgell, A; Mueller, I; Siba, PM; Betuela, I; Davis, TM (2014). “Artemisinin-naphthoquine versus artemether-lumefantrine for uncomplicated malaria in Papua New Guinean children: an open-label randomized trial”. PLoS Med. 11: e1001773. doi:10.1371/journal.pmed.1001773. PMC 4280121
 . PMID 25549086.
. PMID 25549086.
Title: Lumefantrine
CAS Registry Number: 82186-77-4
CAS Name: (9Z)-2,7-Dichloro-9-[(4-chlorophenyl)methylene]-a-[(dibutylamino)methyl]-9H-fluorene-4-methanol
Additional Names: 2-dibutylamino-1-[2,7-dichloro-9-(4-chlorobenzylidene)-9,11-fluoren-4-yl]ethanol; dl-benflumelol; benflumetol; BFL
Manufacturers’ Codes: CPG-56695
Molecular Formula: C30H32Cl3NO
Molecular Weight: 528.94
Percent Composition: C 68.12%, H 6.10%, Cl 20.11%, N 2.65%, O 3.02%
Literature References: Racemic aryl alcohol originally synthesized in the 1970’s by the Academy of Military Medical Sciences in Beijing, China. Inhibits hemozoin formation. Prepn: R. Deng et al., CN 1042535 (1990 to Acad. Military Med. Sci., Microbiol. & Epidemic Dis. Instit.); C.A. 114, 6046 (1991). LC determn in plasma: A. Annerberg et al., J. Chromatogr. B 822, 330 (2005). In vitro activity against Plasmodium falciparum: B. Pradines et al., Antimicrob. Agents Chemother. 43, 418 (1999).
Properties: Odorless, yellow powder. Poorly sol in water, oil, and most organic solvents. Sol in unsaturated fatty acids.
Derivative Type: Co-artemether
CAS Registry Number: 141204-94-6
Manufacturers’ Codes: CPG-56697
Trademarks: Coartem (Novartis); Riamet (Novartis)
Literature References: Fixed 6:1 mixture with artemether, q.v. Clinical pharmacokinetics and bioavailability: F. Ezzet et al., Br. J. Clin. Pharmacol. 46, 553 (1998). Clinical trial in children against P. falciparum malaria: C. Hatz et al., Trop. Med. Int. Health 3, 498 (1998); in adults: S. Looareesuwan et al., Am. J. Trop. Med. Hyg. 60, 238 (1999). Review of comparative clinical trials in malaria: A. A. Omari et al., Trop. Med. Int. Health 9, 192-199 (2004).
Therap-Cat: Antimalarial.
Keywords: Antimalarial.
///////////lumefantrine, lumefantrene, Antimalarial, CPG-56695, CPG 56695, Benflumetol, dl-Benflumelol
CCCCN(CCCC)CC(O)C1=C2C(=CC(Cl)=C1)\C(=C/C1=CC=C(Cl)C=C1)C1=C2C=CC(Cl)=C1































 . PMID 19803708.
. PMID 19803708. . PMID 25549086.
. PMID 25549086.















