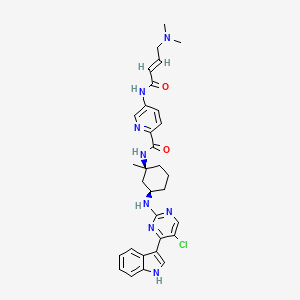
MEVOCICLIB,
CAS 1816989-16-8
SY 1365
N-[(1S,3R)-3-[[5-chloro-4-(1H-indol-3-yl)pyrimidin-2-yl]amino]-1-methylcyclohexyl]-5-[[(E)-4-(dimethylamino)but-2-enoyl]amino]pyridine-2-carboxamide
N-((lS,3R)-3-(5-chloro-4-(lH-indol-3-yl)pyrimidin-2-ylamino)-l-methylcvclohexyl)-5-((E)-4-(dimethylamino)but-2-enamido)picolinamide
HS Tariff Code: 2934.99.9001
Syros
| Molecular Weight |
587.12 |
|---|---|
| Formula |
C₃₁H₃₅ClN₈O₂ |
- OriginatorSyros Pharmaceuticals
- ClassAmides; Amines; Antineoplastics; Chlorinated hydrocarbons; Cyclohexanes; Indoles; Pyridines; Pyrimidines; Small molecules
- Mechanism of ActionCyclin-dependent kinase-activating kinase inhibitors
- DiscontinuedAcute myeloid leukaemia; Breast cancer; Haematological malignancies; Ovarian cancer; Solid tumours
- 23 Oct 2019Discontinued – Preclinical for Haematological malignancies and Acute myeloid leukaemia in the USA (Parenteral); Phase-I for Solid tumours, Ovarian cancer and Breast cancer in the USA (IV) because data obtained did not support an optimal profile for patients and indicated higher or frequent dosing
- 07 Dec 2018Pharmacodynamics data from preclinical trials in Breast cancer presented at the 41st Annual San Antonio Breast Cancer Symposium (SABCS-2018)
- 15 Nov 2018Adverse events, efficacy and pharmacokinetics data from a phase I trial in Solid tumours presented at the 30th EORTC-NCI-AACR Molecular Targets and Cancer Therapeutics Symposium (EORTC-NCI-AACR-2018)
| Clinical Trial |
|
|---|
Mevociclib (SY-1365) is a potent and first-in-class selective CDK7 inhibitor, with a Ki of 17.4 nM. Mevociclib exhibits anti-proliferative and apoptotic effects in solid tumor cell lines. Mevociclib possesses anti-tumor activity in hematological and multiple aggressive solid tumors.
Mevociclib, also known as SY-1365, is a CDK7 inhibitor. In vitro, SY-1365 inhibited cell growth of many different cancer types at nanomolar concentrations. SY-1365 treatment decreased MCL1 protein levels, and cancer cells with low BCL-XL expression were found to be more sensitive to SY-1365. Transcriptional changes in acute myeloid leukemia (AML) cell lines were distinct from those following treatment with other transcriptional inhibitors. SY-1365 demonstrated substantial anti-tumor effects in multiple AML xenograft models as a single agent; SY-1365-induced growth inhibition was enhanced in combination with the BCL2 inhibitor venetoclax.
Syn
WO2015154038
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015154038
Example 16. Synthesis of N-((lS,3R)-3-(5-chloro-4-(lH-indol-3-yl)pyrimidin-2-ylamino)-l-methylcvclohexyl)-5-((E)-4-(dimethylamino)but-2-enamido)picolinamide (Compound 267).
[251] (+/-) Benzyl tert-butyl ((lS,3R)-l-methylcvclohexane-l,3-diyl)dicarbamate
(+/-)
[252] A solution of (+/-)-(lS,3R) -3-((ieit-¾itoxycarbonyl)amino)–l -raethylcyclohexanecarboxylic acid prepared as in WO2010/148197 (4.00 g, 15.5 mmol) in toluene (Ϊ 55 mL) was treated with Et3N (2.4 mL, 17.1 mmol) and DPPA (3.68 mL, Ϊ7.1 mmol) and heated at reflux for lh. Benzyl alcohol (8.0 mL, 77.7 mmol) and Et3N (4.4 mL , 31 .4 mmol) were added to the reaction mixture and the solution was heated at 100 °C for 72h. The mixture was cooled to room temperature and then diluted with EtOAc (300 mL) and H20 (300 mL). The layers were separated and the aqueous layer was extracted with EtOAc (3 x 200 mL). The combined organics layers were washed with brine (100 mL), filtered and evaporated to dryness. The residue was purified by Si02 chromatography (EtOAc in hexanes, 0 to 50% gradient) and afforded the title compound (3.40 g, 9.38 mmol, 60%) as a white solid.
[253] Benzyl tert-butyl ((lS,3R)-l-methylcvclohexane-l,3-diyl)dicarbamate and benzyl tert- -l-methylcvclohexane-l,3-diyl)dicarbamate
(+/-)
[254] Both enantiomers of (+/-)-Benzyl tert-butyl ((lS,3R)-l-methylcyclohexane-l,3-diyl)dicarbamate (3.40 g, 9.38 mmol) were separated using preparative chiral HPLC (Chiralpak IA, 5 urn, 20×250 mm; hex/MeOH/DCM = 90/5/5) to yield both compounds benzyl tert-butyl ((lS,3R)-l-methylcyclohexane-l,3-diyl)dicarbamate (1.20 g, 3.31 mmol) and benzyl iert-butyl ((lR,3S)-l-methylcyclohexane-l,3-diyl)dicarbamate (1.15 g, 3.17 mmol) as white solids.
255 Benzyl ((lS,3R)-3-amino-l-methylcvclohexyl)carbamate hydrochloride
[256] A solution of benzyl tert-butyl ((lS,3R)-l-methylcyclohexane-l,3-diyl)dicarbamate (700 mg, 1.93 mmol) in DCM (19 mL) was treated with a 4M solution of HCI in dioxane (9.66 mL, 38.6 mmol) and stirred 16h at rt. The mixture was evaporated to dryness and afforded the title compound (577 mg, 1.93 mmol, 100%) as a white solid which was used in the next step without further purification.
[257] (lS,3R)-Benzyl-3-(5-chloro-4-(l-(phenylsulfonyl)-lH ndol-3-yl)pyrimidin-2-ylamino)-1-methylcyclohexylcarbamate
[258] A solution of 3-(2,5-dichloropyrimidin-4-yl)-l-(phenylsulfonyl)-lH-indole (1.02 g, 2.53 mmol), benzyl (( iS,3 )-3- amino- 1 -methylcyclohexyljcarbaniaie hydrochloride (577 mg, 1.93 mmol) and DIPEA (1.15 mL, 6.60 mmol) in NMP (11 mL) was heated at 135 °C (microwave) for 60 min. The cooled mixture was diluted with EtOAc (250 mL), washed with H20 (100 mL), brine (100 mL), dried over MgS04, filtered and evaporated to dryness. The residue was purified by Si02 chromatography (EtOAc in DCM, 0 to 50% gradient) and afforded the title compound (747 mg, 1.19 mmol, 54%) as a yellow foam.
[259] (lS,3R)-N-(5-chloro-4-(l-(phenylsulfonyl)-lH ndol-3-yl)pyrimidin-2-yl)-3-methylcvclohexane-l,3-diamine
[260] A cooled (-78°C) solution of (lS,3R)-benzyl-3-(5-chloro-4-(l-(phenylsulfonyl)-lH-indol-3-yl)pyrimidin-2-ylamino)-l-methylcyclohexylcarbamate (747 mg, 1.19 mmol) in DCM (39 mL) was treated with a 1M solution of BBr3 in DCM (2.83 mL, 2.83 mmol) and was slowly warmed up to rt. MeOH (10 mL) was added to the mixture was the resulting solution was stirred lh at rt. The resulting mixture was evaporated to dryness. The residue was purified by reverse phase chromatography (C18, H20/ACN +0.1% HC02H, 0 to 60% gradient) and afforded the title compound (485 mg, 0.978 mmol, 83%) as a yellow solid.
[261] 5-amino-N-( ( lS,3R)-3-( 5-chloro-4-(l-(phenylsulfonyl)-lH-indol-3-yl)pyrimidin-2-ylamino)-l-methylcvclohexyl)picolinamide
[262] A solution of (lR,3S)-N-(5-chloro-4-(l-(phenylsulfonyl)-lH-indol-3-yl)pyrimidin-2-yl)-3-methylcyclohexane-l,3-diamine (75.0 mg, 0.150 mmol) and 5-aminopicolinic acid (25.0 mg, 0.180 mmol) in DMF (5.0 mL) was treated with HBTU (86.0 mg, 0.230 mmol) and DIPEA (79 μί, 0.45 mmol). The resulting mixture was stirred 5h at rt and diluted with MeTHF (50 mL) and saturated NaHC03 (50 mL). The layers were separated and the aqueous layer was extracted with MeTHF (2 x 50 mL). The combined organic layers were dried over MgS04, filtered and evaporated to dryness. The residue was purified by Si02 chromatography (EtOAc in DCM, 0 to 50% gradient) and afforded the title compound (74.0 mg, 0.120 mmol, 79%) as a light yellow oil.
[263] 5-amino-N-((lS,3R)-3-(5-chloro-4-(lH ndol-3-yl)pyrimidin-2-ylamino)-l-methylcyclohexyDpicolinamide
[264] A solution of 5-amino-N-((lS,3R)-3-(5-chloro-4-(l-(phenylsulfonyl)-lH-indol-3-yl)pyrimidin-2-ylamino)-l-methylcyclohexyl)picolinamide (74.0 mg, 0.120 mmol) in 1,4-dioxane (4.0 mL) was treated with a 2M solution of NaOH in H20 (960 μί, 4.78 mmol) and heated at 60°C for lh. The cooled mixture was diluted with MeTHF (30 mL) and H20 (30 mL). The layers were separated and the aqueous layer was extracted with MeTHF (3 x 30 mL). The combined organic layers were dried over MgS04, filtered and evaporated to dryness affording the title compound (57.0 mg, 0.120 mmol, 100%) as a light yellow oil which was used in the next step without further purification.
[265] N-((lS,3R)-3-(5-chloro-4-(lH ndol-3-yl)pyrimidin-2-ylamino)-l-methylcvd^
( ( E)-4-(dimethylamino)but-2-enamido )picolinamide ( Compound 267)
[266] A cooled (-78°C) solution of 5-amino-N-((lS,3R)-3-(5-chloro-4-(lH-indol-3-yl)pyrimidin-2-ylamino)-l-methylcyclohexyl)picolinamide (57.0 mg, 0.120 mmol) and DIPEA (104 0.598 mmol) in THF/NMP (4.0 mL/1.0 mL) was treated with a 54.2 mg/mL solution of (E)-4-bromobut-2-enoyl chloride in DCM (104 μί, 0.598 mmol). The resulting mixture was stirred 4h at -78°C before addition of a 2M solution of dimethylamine in THF (359 μί, 0.717 mmol). The resulting mixture was warmed up to rt and stirred 45min at this temperature before being evaporated to dryness. The residue was purified by reverse phase chromatography (C18, H20/ACN +0.1% HC02H, 0 to 50% gradient) and afforded the title compound (15.0 mg, 0.026 mmol, 22%) as a white solid after lyophilization. LCMS: Calculated: 587.12; Found (M+H+): 587.39. 1H NMR (500 MHz, DMSO) δ 11.84 (s, 1H), 10.54 (s, 1H), 8.82 (d, J = 2.3 Hz, 1H), 8.64 (s, 1H), 8.47 (s, 1H), 8.25 (dd, J = 8.6, 2.4 Hz, 2H), 7.98 (d, J = 8.9 Hz, 2H), 7.50 (d, J = 7.7 Hz, 1H), 7.25 – 7.07 (m, 3H), 6.81 (dt, J = 15.5, 5.8 Hz, 1H), 6.29 (d, J = 15.4 Hz, 1H), 4.23 – 4.08 (m, 1H), 3.08 (dd, J = 5.7, 1.1 Hz, 2H), 2.46 – 2.37 (m, 1H), 2.18 (s, 6H), 2.04 – 1.95 (m, 2H), 1.87 – 1.70 (m, 3H), 1.63 – 1.46 (m, 4H), 1.39 – 1.26 (m, 1H).
Ref
- [1]. Hu S, et al. Discovery and characterization of SY-1365, a selective, covalent inhibitor of CDK7. Cancer Res. 2019 May 7.[2]. Shanhu Hu, et al. SY-1365, a potent and selective CDK7 inhibitor, exhibits promising anti-tumor activity in multiple preclinical models of aggressive solid tumors.
///////////////MEVOCICLIB, 1816989-16-8, SY 1365
CN(C)C\C=C\C(=O)Nc1ccc(nc1)C(=O)N[C@]1(C)C[C@@H](CCC1)Nc1ncc(Cl)c(n1)c1c[NH]c2ccccc21