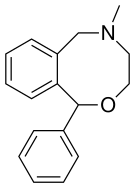
Nefopam
- Molecular Formula C17H19NO
- Average mass 253.339 Da
Wound-Healing Agents
Nefopam, sold under the brand names Acupan among others, is a painkilling medication. It is primarily used to treat moderate, acuteor chronic pain. [3]
It is believed to work in the brain and spinal cord to relieve pain. There it is believed to work via rather unique mechanisms. Firstly it increases the activity of the serotonin, norepinephrine and dopamine, neurotransmitters involved in, among other things, pain signaling. Secondly, it modulates sodium and calcium channels, thereby inhibiting the release of glutamate, a key neurotransmitter involved in pain processing.[4
Medical uses
Nefopam has additional action in the prevention of shivering, which may be a side effect of other drugs used in surgery.[5] Nefopam was significantly more effective than aspirin as an analgesic in one clinical trial,[6] although with a greater incidence of side effects such as sweating, dizziness and nausea, especially at higher doses.[7][8] Nefopam is around a third to half the potency and slightly less effective as an analgesic compared to morphine,[9][10][11] or oxycodone,[12] but tends to produce fewer side effects, does not produce respiratory depression,[13] and has much less abuse potential, and so is useful either as an alternative to opioids, or as an adjunctive treatment for use alongside opioid(s) or other analgesics.[11][14] Nefopam is also used to treat severe hiccups.[15]
Contraindications
Nefopam is contraindicated in people with convulsive disorders, those that have received treatment with irreversible monoamine oxidase inhibitors such as phenelzine, tranylcypromine or isocarboxazid within the past 30 days and those with myocardial infarctionpain, mostly due to a lack of safety data in these conditions.[16]
Side effects
Common side effects include nausea, nervousness, dry mouth, light-headedness and urinary retention.[16] Less common side effects include vomiting, blurred vision, drowsiness, sweating, insomnia, headache, confusion, hallucinations, tachycardia, aggravation of angina and rarely a temporary and benign pink discolouration of the skin or erythema multiforme.[16]
Overdose
Overdose and death have been reported with nefopam,[17] although these events are less common with nefopam than with opioid analgesics.[18] Overdose usually manifests with convulsions, hallucinations, tachycardia, and hyperdynamic circulation.[16] Treatment is usually supportive, managing cardiovascular complications with beta blockers and limiting absorption with activated charcoal.[16]
Interactions
It has additive anticholinergic and sympathomimetic effects with other agents with these properties.[16] Its use should be avoided in people receiving some types of antidepressants (tricyclic antidepressants or monoamine oxidase inhibitors) as there is the potential for serotonin syndrome or hypertensive crises to result.[16]
Pharmacology
| Site | Ki (nM) |
|---|---|
| SERT | 29 |
| NET | 33 |
| DAT | 531 |
| 5-HT2A | 1,685 |
| 5-HT2B | 330 |
| 5-HT2C | 56 |
The mechanism of action of nefopam and its analgesic effects are not well understood, although inhibition of the reuptake of serotonin, norepinephrine, and to a lesser extent dopamine (that is, acting as an SNDRI) is thought to be involved.[21][4] It also reduces glutamate signaling via modulating sodium and calcium channels.[22][4]
Pharmacokinetics
The absolute bioavailability of nefopam is low.[1] It is reported to achieve therapeutic plasma concentrations between 49 and 183 nM.[20] The drug is approximately 73% protein-bound across a plasma range of 7 to 226 ng/mL (28–892 nM).[1] The metabolism of nefopam is hepatic, by N–demethylation and via other routes.[1] Its terminal half-life is 3 to 8 hours, while that of its active metabolite, desmethylnefopam, is 10 to 15 hours.[1] It is eliminated mostly in urine, and to a lesser extent in feces.[1]
Chemistry
Nefopam is a cyclized analogue of orphenadrine, diphenhydramine, and tofenacin, with each of these compounds different from one another only by the presence of one or two carbons.[23][24][25] The ring system of nefopam is a benzoxazocine system.[23][26]
Society and culture
Recreational use
Recreational use of nefopam has been reported,[17] although this is less common than with opioid analgesics.[18]
SYNTHESIS

PATENT
ES 8605495
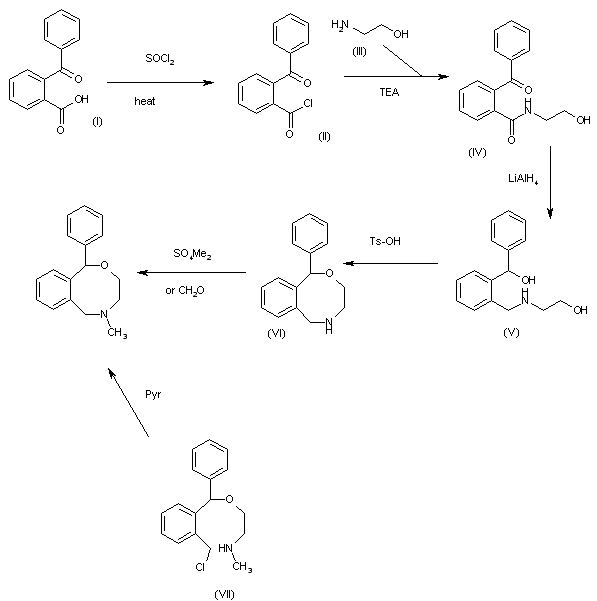
The reaction of 2-benzoylbenzoic acid (I) with SOCl2 in CHCl3, benzene or DMF gives the corresponding acyl chloride (II), which is condensed with ethanolamine (III) by means of TEA in CHCl3 to yield the amide (IV). The reduction of (IV) with LiAlH4 in THF affords the diol (V), which is cyclized by means of Ts-OH in refluxing benzene to provide 1-phenyl-3,4,5,6-tetrahydro-1H-2,5-benzoxazocine (VI). Finally, this compound is methylated by means of dimethyl sulfate in refluxing benzene, or by means of formaldehyde in hot dioxane/water. Alternatively, the cyclization of N-[2-[1-[2-(chloromethyl)phenyl]-1-phenylmethoxy]ethyl]-N-methylamine (VII) by means of pyridine in refluxing acetonitrile gives also the target benzoxazocine
PATENT
KE 8201564
PATENT
ES 8104800
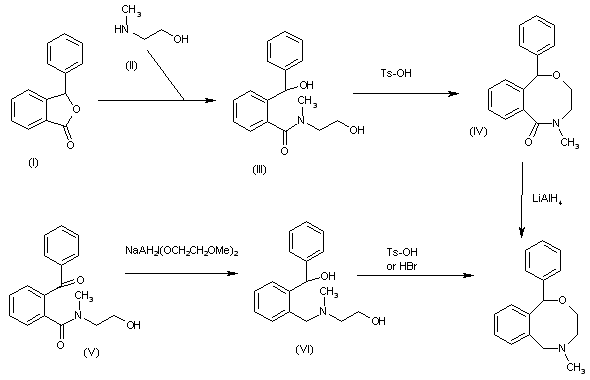
The reaction of 3-phenylphthalide (I) with N-methylethanolamine (II) in refluxing benzene gives N-(2-hydroxyethyl)-2-(1-hydroxy-1-phenylmethyl)-N-methylbenzamide (III), which is cyclized by means of Ts-OH in refluxing toluene to yield 5-methyl-1-phenyl-3,4,5,6-tetrahydro-1H-2,5-benzoxazocin-6-one (IV). Finally this compound is reduced with LiAlH4 in refluxing THF to afford the target benzoxazocine. In an alternative method, the reduction of 2-benzoyl-N-(2-hydroxyethyl)-N-methylbenzamide (V) by means of sodium bis(2-methoxyethoxy)aluminum hydride in refluxing toluene gives the diol (VI), which is then cyclized by means of Ts-OH in refluxing toluene, or by means of aq. 48% HBr in hot chloroform to afford the target benzoxazocine
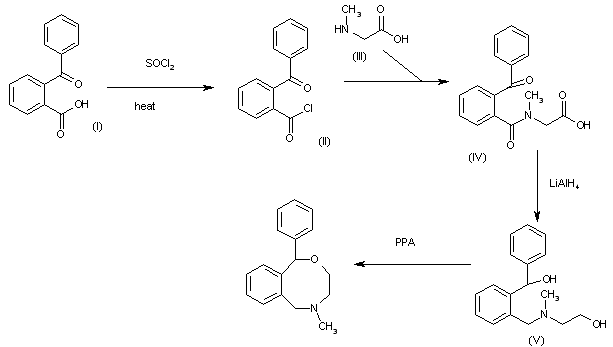
The reaction of 2-benzoylbenzoic acid (I) with refluxing SOCl2 gives the corresponding acyl chloride (II), which is condensed with 2-(methylamino)acetic acid (III) in benzene to yield the N-(2-benzoylbenzoyl)-N-methylglycine (IV). The reduction of (IV) by means of LiAlH4 in refluxing THF affords the diol (V), which is finally cyclized by means of PPA at 80 C to provide the target benzoxazocine.
PATENT
US 4208349
PATENT
https://www.google.com/patents/EP0033585A1?cl=en
This compound is useful as an intermediate in producing the pharmacologically valuable 3,4,5,6-tetrahydro-5-methyl-l-phenyl-lH-2,5-benzoxazocine- hydrochloride, or nefopam, which is used, e.g. as a muscle relaxant, an analgesic or antidepressant drug.
Processes for producing the compound of formula I are already known. For instance, according to German Patent 1,620,198, phthalic aldehyde is used as a starting material. According to the German Patent, the phthalic aldehyde is reacted with a Grignard reagent, phenylmagnesiumbromide, and an N-substituted aminoalcohol is coupled to the reaction mixture, to produce a product of formula:
This product is catalytically hydrogenated with the aid of Pd/C, Pt or Raney-Ni, and a product of formula I is obtained.
In another method, according to the German Patent 1,620,198, o-benzoylbenzoic acid is used as a starting material, which is converted by means of thionylchloride into an acid chloride. To this acid chloride is then coupled methylethanolamine, and N-(2-hydroxyethyl)-N-methyl-o-benzoylbenzamide is obtained as an intermediate, which is reduced using LiAlH4 and an end-product of formula I is produced.
According to United States Patent 3,487,153 o-benzoylbenzoic acid amide is used as starting material to produce the intermediate. With the aid of thionylchloride the corresponding acid chloride is formed, which is allowed to react with N-methyl-2-aminoethanol. The so-produced N-(2-hydroxyethyl)-N-methyl-o-benzoylbenzamide is reduced with LiAlH4 to 2{[N-(2-hydroxyethyl)-N-methyl)amino}-methylbenzhydrol.
According to German Offenlegungschrift 2,834,312 o-benzoylbenzoic acid is used as a starting material, which is allowed to react with phosphorus trichloride in dichloroethane. The acid chloride formed is allowed to react with triethylamine and N-methyl-2-hydroxyethyl- amine, after which N-(2-hydroxyethyl)-N-methyl-o-benzoylbenzamide is formed. This compound is treated with phosphorus trichloride (at pH=7.0) and N-(2-chloroethyl)-N-methyl-o-benzoylbenzoic amide is obtained, which is then reduced with NaBH4 in acetic acid. By these means 2-{[N-(2-hydroxyethyl)-N-methyl]-amino?-methylbenzhydrol is obtained.
According to Finnish Patent No. 54793, which corresponds to Canadian Patent 982,608, a compound of formula III is used as starting material, which is reduced with NaBH4 to a corresponding benzhydrol derivative of formula IV, which is then allowed to react with an alkylamine to an a-substituted 2-aminomethyl- benzylalcohol of formula V. The abovementioned Patent does not concern either the preparation of nefopam or its intermediates
When reviewing the abovementioned Patents, i.e. German Patent 1,620,198 and United States Patent 3,487,153, one can observe the disadvantage that catalytic hydrogenation with palladium on charcoal, platinum or Raney-Ni, or lithium aluminium hydride are to be used to reduce the starting materials. This latter reagent is expensive and reacts with water very intensely, so that even a little humidity in the working surroundings or in the solvents can cause a fire. Explosive hydrogen is also produced by the reaction. Grignard reactions and catalytic hydrogenations are technically difficult to perform on a large scale. Moreover, the price of o-phthalic aldehyde is high.
According to the method described in German Offenlegungschrift 2,834,312 the reducing of the amide- carbonyl group with sodium borohydride in acetic acid requires, however, great additional amounts or about 2-3 equivalents of sodium borohydride. The yield of the reaction is quite poor (about 50-55%) and the reaction time is long, so the production costs become high. Moreover, the number of synthetic reaction steps is high and the use of phosphorus trichloride especially on a production scale is difficult.
In the method according to the Finnish Patent 54793, which corresponds to the Canadian Patent 982,608, a benzophenone derivative (of formula III) is reduced with NaBH4 to the corresponding benzhydrol derivative (formula IV). This compound is, however, unstable because of the methylene halogen group in o-position, especially when R1 = H in formula IV. On storing for only a short time hydrogenchloride gas is released and a very stable 5-ring ether is formed, which is useless. The use of this method on a large scale is therefore almost impossible, because the intermediate is impossible to isolate fast enough to obtain at least a reasonable amount of the end product.
The present invention provides a process for the preparation of 2-{[N-(2-hydroxyethyl)-N-methyl]-amino}-methylbenzhydrol (as such or as an acid addition salt) which comprises reacting 2-chloromethylbenzophenone with 2-methylaminoethanol to give 2-J[N-(2-hydroxyethyl)-N-methyl]-amino}-methylbenzophenone (as such or as a salt), and reducing the latter with sodium borohydride to give 2-{[N-2-(hydroxyethyl)-N-methyl)-aminol}-methylbenzhydrol (as such or as an acid addition salt). The 2-chlorobenzophenone (of formula VI) is brought to react with methylethanolamine in the presence of e.g. sodium carbonate, and 2-{[N-(2-hydroxyethyl)-N-methyl]-amino}- methylbenzophenone (of formula VII) is formed. This substance is theoreduced with sodium borohydride to 2-{(N-(hydroxyethyl)-N-methyl]-amino}-methylbenzhydrol (of formula VIII), as shown below:
The starting material, 2-chloromethyl benzophenone, can be produced in known manner by halogenating the corresponding 2-methylbenzophenone (Monatshefte far Chemie 99, 1990-2003, 1968) or 2-hydroxymethylbenzophenone, of which the former is commercially available and the latter can be produced in known manner from the phthalide (see British Patent 1,526,331). The compound of formula VII is new, and as such a feature of the invention.
The following Examples illustrate the invention.
EXAMPLE 1
8.50 g (0.037 mol) 2-chloromethylbenzophenone is dissolved in 40 ml ethylalcohol, and 4.0 g sodium carbonate and 2.80 g (0.037 mol) 2-methylaminoethanol are added, The mixture is boiled for 3 hours and the salts formed are filtered off from the cooled solution. A pure reaction product is obtained when the ethanol is evaporated from the solution and the product is crystallized as a hydrochloride salt from a mixture of diethylether and alcohol. The yield is 10.7 g (95 %) of 2{(N-(2-hydroxyethyl)-N-methyl]-amino}- methylbenzophenone as a crystalline powder, m.p. 135-136 C.
This compound, as the free base, shows the following N M R spectrum (in cDC13 using T M S as internal reference): 7.8 – 7.1 (aromatic), 3.5 (singlet), 3.4 (triplet), about 2.6 (singlet), 2.3 (triplet),1.9 (singlet). Its infra-red spectrum shows maxima at the following frequencies (cm-1): 680, 720, 760, 910, 1010, 1060, 1140, 1230, 1260, 1300, 1430, 1560,1580, 1640, 2760, 2920, 3030 and 3400.
EXAMPLE 2
10.0 g (0.033 mol) of the hydrochloride salt prepared in Example 1 are dissolved in a mixture comprising 15 ml water, 60 ml methanol and 3.5 g sodium hydroxide. To the mixture is added 0.65 g sodium borohydride and the solution is mixed for half an hour at room temperature.
The solution is acidified with concentrated hydrochloric acid and the methanol is evaporated in vacum. 40 ml of water is added, the pH of the water solution is adjusted with diluted sodium hydroxide solution to an alkaline reaction and the product is extracted into chloroform. The chloroform extracts are washed well with water, dried over sodium sulphate and evaporated to dryness. The product is separated by precipitating as a hydrochloride salt from a mixture of diethylether and ethylalcohol. The yield is 9.8 g (96 %) of 2-{(N-(2-hydroxyethyl)-N-methyl]-amino}- methylbenzhydrol as a crystalline powder, m.p. 128-133 C.
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| DE2834312A1 * | Aug 4, 1978 | Feb 15, 1979 | Riker Laboratories Inc | Verfahren zur herstellung von 2 eckige klammer auf n-(2-hydroxyaethyl)- n-niederalkylaminomethyl eckige klammer zu -benzhydrolen |
| ES485471A * | Title not available |
| Reference | ||
|---|---|---|
| 1 | * | CHEMICAL ABSTRACTS Vol. 94, No. 11, 16 March 1981 Columbus, Ohio, USA FARMA-LEPORI “2-(n-2-Hydroxyethylmethylaminomethyl)benzhydrol” page 690, column 2, Abstract No. 83757s & ES – A – 485 471. |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| CN102363610A * | Nov 1, 2011 | Feb 29, 2012 | 安徽万和制药有限公司 | New method for synthesizing nefopam hydrochloride |
| CN102924320A * | Nov 15, 2012 | Feb 13, 2013 | 南京海陵中药制药工艺技术研究有限公司 | Method for preparing nefopam intermediate I |
| CN102924320B * | Nov 15, 2012 | Jan 14, 2015 | 南京海陵中药制药工艺技术研究有限公司 | Method for preparing nefopam intermediate I |
PATENT
CN 102363610
https://www.google.com/patents/CN102363610A?cl=en
Example 1:
[0043] o-benzoyl benzoate 120g, phosphorus trichloride 30g, 220g of the mixture placed in a reaction flask dichloroethane, Mh was stirred at room temperature, the supernatant was separated to give acid chloride solution A;
[0044] A solution of this acid chlorine solution to 5 ° C and at a pre-filled with N- methyl ethanolamine 44g, triethylamine 64g, 200g dichloroethane reaction flask, stirred at room temperature drop after 10h, get amine solution B;
[0045] B in the amine solution and then dropping phosphorus trichloride 33g, reaction at 65 ° C 2h, washed with water cooling, the solution was washed with a dilute solution of sodium hydroxide, to sub-alkaline layer chloride solution C.
[0046] In the reaction flask was added a certain amount of potassium borohydride; potassium borohydride to mass, and then the mixture was added 15% acetic acid and dichloroethane (solvent of acetic acid mass ratio of 1: 1); to potassium borohydride mass, and then added dropwise to obtain 45% of the chlorination reaction chloride solution C, stirring the reaction was heated to reflux for 2h, pre-reduction; with potassium borohydride mass, further addition of 10% acetic acid and dichloroacetyl alkane mixture (mass ratio of acetic acid to solvent is 1: 1), the reaction was stirred Ih; in reducing mass, and finally the mixture was added dropwise 45% obtained by chlorinating liquid the chlorination reaction C with acetic acid (chloride quality liquid C and acetic acid ratio of 1: 1), the reaction was stirred tank for the final reduction. Plus 40% hydrolyzed sodium hydroxide solution, the organic layer was separated D
[0047] The separated organic layer D was cooled to room temperature and added slowly to 65 ° C hydrobromide reaction 6h, the reaction is completed, cooled to 0 ° C, and filtered to give the cyclization product E.
[0048] The cyclization to give the reaction product E was added sodium hydroxide solution and then dropwise addition of concentrated hydrochloric acid, to obtain Nefopam.
[0049] Example 2:
[0050] o-benzoyl benzoate 120g, phosphorus trichloride 30g, 220g of the mixture placed in a reaction flask dichloroethane, Mh was stirred at room temperature, the supernatant was separated to give acid chloride solution A;
[0051] A solution of this acid chlorine solution to 5 ° C and at a pre-filled with N- methyl ethanolamine 44g, triethylamine 64g, 200g dichloroethane reaction flask, stirred at room temperature drop after 10h, get amine solution B;
[0052] B in the amine solution and then dropping phosphorus trichloride 33g, reaction at 65 ° C 2h, washed with water cooling, the solution was washed with a dilute solution of sodium hydroxide, to sub-alkaline layer chloride solution C.
[0053] In the reaction flask was added a certain amount of potassium borohydride; potassium borohydride to mass, and then the mixture was added 25% acetic acid and dichloroethane (solvent of acetic acid mass ratio of 1: 1); to potassium borohydride mass, then dropping to 50% of the chlorination reaction chloride solution C, stirring heated to reflux for 2h, pre-reduction; potassium borohydride mass, then add 20% acetic acid and dichloroethane alkane mixture (mass ratio of acetic acid to solvent is 1: 1), the reaction was stirred Ih; in reducing mass, and finally the mixture was added dropwise a 50% solution chlorination reaction C and obtained by chlorinating acetic acid (chloride quality liquid C and acetic acid ratio of 1: 1), the reaction was stirred tank for the final reduction. Plus 40% hydrolyzed sodium hydroxide solution, the organic layer was separated D
[0054] The separated organic layer D was cooled to room temperature and added slowly with stirring at 65 ° C the reaction hydrobromide 8h, the reaction is completed, cooled to 0 ° C, and filtered to give the cyclization product E.
[0055] The cyclization to give the reaction product E was added sodium hydroxide solution and then dropwise addition of concentrated hydrochloric acid, to obtain Nefopam.
[0056] The applicant stated the above embodiments of the present invention will be described in detail the process equipment and process of the present invention, but the invention is not limited to the above detailed process equipment and process, that does not mean that the present invention must rely on such details process equipment and processes to be implemented. Skill in the art should be appreciated that any improvement in the present invention, the present invention is the product of the raw materials equivalents and adding auxiliary components, choice of specific ways, and fall within the scope of the public of the scope of the present invention.



| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| EP0033585A1 * | Jan 9, 1981 | Aug 12, 1981 | Farmos-Yhtyma Oy | A process for the preparation of a benzhydrol derivative and a novel intermediate for use therein |
| US3978085 * | Mar 7, 1975 | Aug 31, 1976 | Riker Laboratories, Inc. | Process for benz[f]-2,5-oxazocines |
| US4208349 * | Mar 5, 1979 | Jun 17, 1980 | Riker Laboratories, Inc. | Process for the preparation of 2-[N-(2-hydroxyethyl)-N-lower alkylaminomethyl]benzhydrols |
| Reference | ||
|---|---|---|
| 1 | * | 胡颂凯: “镇痛药盐酸苯并噁唑辛的合成“, 《医药工业》, no. 8, 28 August 1984 (1984-08-28) |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| CN102924320A * | Nov 15, 2012 | Feb 13, 2013 | 南京海陵中药制药工艺技术研究有限公司 | Method for preparing nefopam intermediate I |
CLIP
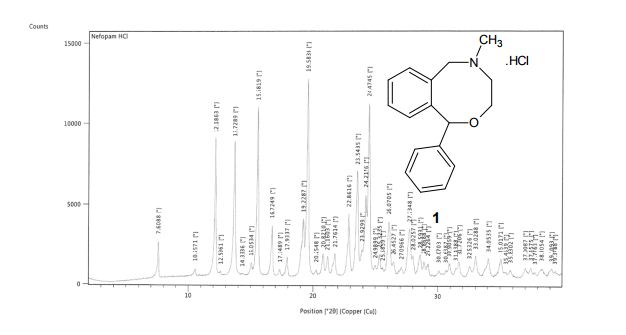
1H NMR (400 MHz, D2O, δ/ppm): 7.36–7.25 (m, 6H, arom H), 7.21–7.18 (m, 2H, arom H), 7.12–7.10 (m, 1H, arom H), 5.89 (s, 1H, Aryl–CH–Aryl), 5.45 (d, 1H, Aryl–CH(H)–N–, J = 12.8 Hz), 4.34–4.27 (m, 1H, –CH(H)–O–), 4.21 (d, 1H, Aryl–CH(H)–N–, J = 13.2 Hz), 4.05–4.00 [m (dt), 1H, –CH(H)–O–, J = 6.8 Hz and J = 3.6 Hz], 3.30-3.23 (m, 1H, –CH(H)– N–), 3.08–3.02 [m (dt), 1H, –CH(H)–N–, J = 7.2 Hz and J = 3.6 Hz), 2.87 (s, 3H, –CH3).
13C NMR (100 MHz, D2O, δ/ppm): 142.4, 141.1, 134.3, 130.5, 129.1, 129.0 (2C), 128.7, 128.4, 127.7 (2C), 125.3, 85.3, 64.9, 58.3, 50.5, 41.6
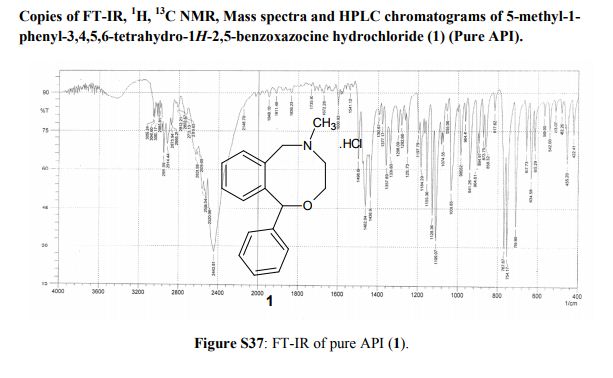
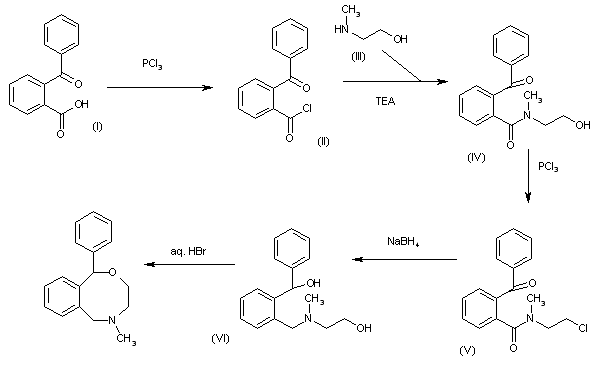
Powder XRD spectra and data of pure API (1). ABOVE
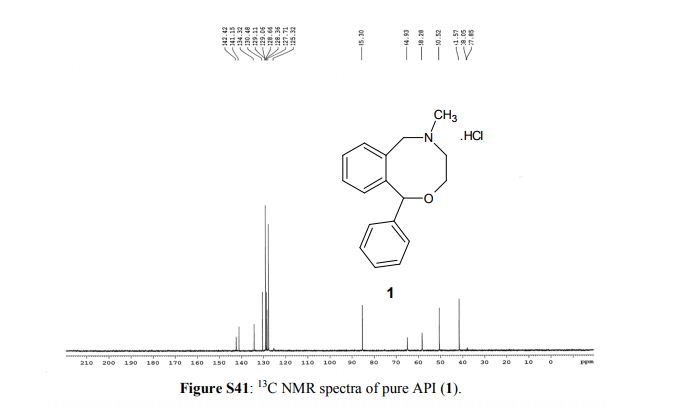
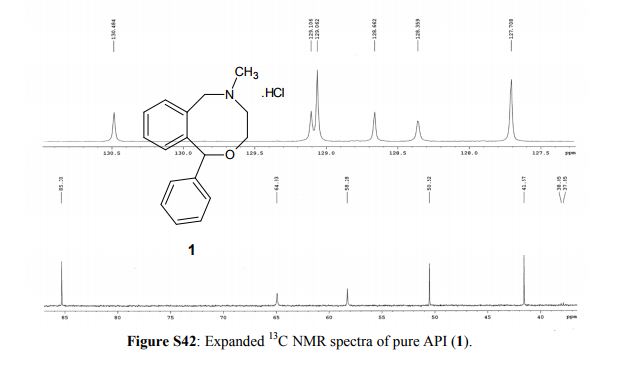
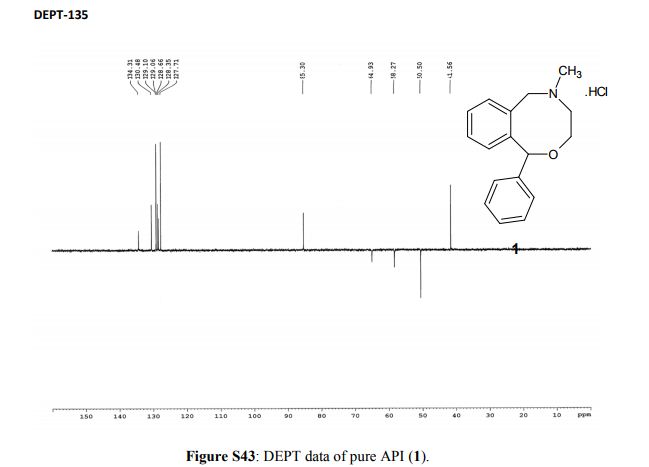
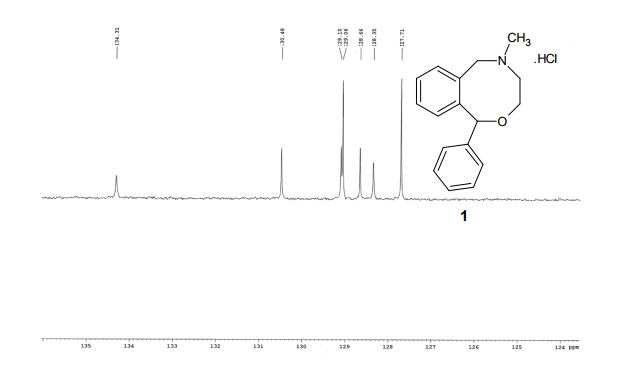
EXPANDED VIEW
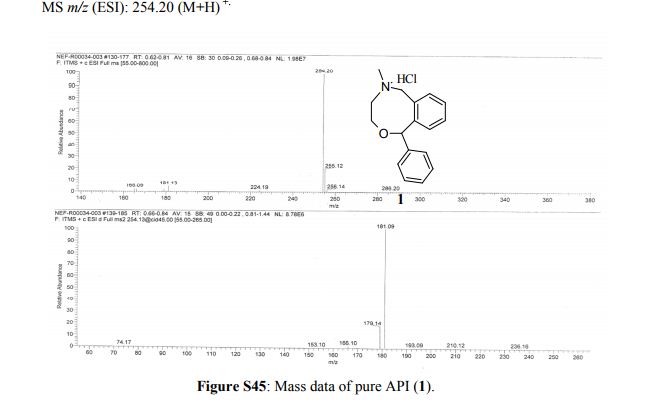
5-Methyl-1-phenyl-3,4,5,6-tetrahydro-1H-2,5-benzoxazocine Hydrochloride (1)
PAPER
Old is Gold? Nefopam Hydrochloride, a Non-opioid and Non-steroidal Analgesic Drug and Its Practical One-Pot Synthesis in a Single Solvent for Large-Scale Production
Mohan Reddy Bodireddy, Kiran Krishnaiah, Prashanth Kumar Babu, Chaithanya Bitra, Madhusudana Rao Gajula*, and Pramod Kumar*
Chemical Research Division, API R&D Centre, Micro Labs Ltd., Plot No.43-45, KIADB Industrial Area, Fourth Phase, Bommasandra-Jigani Link Road, Bommasandra, Bangalore-560 105, Karnataka, India
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00228
*Tel.: 0811 0415647, ext. 245; + 91 9008448247 (mobile). E-mail: pramodkumar@microlabs.in., *E-mail: gmadhusudanrao@yahoo.com.

Nefopam hydrochloride is extensively used in most of the European countries until today as an analgesic because of its non-opiate (non-narcotic) and non-steroidal action with fewer side effects compared with opioid and other analgesics, which cause more troublesome side effects. A multikilogram synthesis of nefopam hydrochloride has been achieved in one pot using a single solvent (toluene). A ≥99.9% purity of the active pharmaceutical ingredient (API) was achieved in excellent overall yield (≥79%). The one-pot, five-step synthetic process involves formation of an acid chloride (3) from benzoylbenzoic acid (2) followed by amidation (4), reduction (5), cyclization (6), and formation of the hydrochloride salt (1). The major advantages include (i) use of a single solvent, (ii) >90% conversion in each step, (iii) a cost-effective and operationally friendly process, (iv) averting the formation of genotoxic impurities, and (v) improved overall yield (≥79%) provided by the one-pot operation. For the first time, we report the characterization data of API 1, intermediates 3, 4, and 5, and also a possible impurity (5a).
CLIP
References
- ^ Jump up to:a b c d e f g h i j k l m Sanga M, Banach J, Ledvina A, Modi NB, Mittur A (2016). “Pharmacokinetics, metabolism, and excretion of nefopam, a dual reuptake inhibitor in healthy male volunteers”. Xenobiotica. 46 (11): 1001–16. PMID 26796604. doi:10.3109/00498254.2015.1136989.
- Jump up^ G. Seyffart (6 December 2012). Drug Dosage in Renal Insufficiency. Springer Science & Business Media. pp. 407–. ISBN 978-94-011-3804-8.
- Jump up^ Brayfield, A, ed. (27 October 2016). “Nefopam hydrochloride”. MedicinesComplete. London, UK: Pharmaceutical Press. Retrieved 4 September 2017.
- ^ Jump up to:a b c Girard, P; Chauvin, M; Verleye, M (January 2016). “Nefopam analgesia and its role in multimodal analgesia: A review of preclinical and clinical studies.”. Clinical and Experimental Pharmacology & Physiology. 43 (1): 3–12. PMID 26475417. doi:10.1111/1440-1681.12506.
- Jump up^ Alfonsi P, Adam F, Passard A, Guignard B, Sessler DI, Chauvin M (January 2004). “Nefopam, a Non-sedative Benzoxazocine Analgesic, Selectively Reduces the Shivering Threshold”. Anesthesiology. 100 (1): 37–43. PMC 1283107
 . PMID 14695722. doi:10.1097/00000542-200401000-00010.
. PMID 14695722. doi:10.1097/00000542-200401000-00010. - Jump up^ Cohen A, Hernandez CM (1976). “Nefopam hydrochloride: new analgesic agent”. Journal of International Medical Research. 4 (2): 138–43. PMID 799984.
- Jump up^ Wang RI, Waite EM (July 1979). “The clinical analgesic efficacy of oral nefopam hydrochloride”. Journal of Clinical Pharmacology. 19 (7): 395–402. PMID 479385. doi:10.1002/j.1552-4604.1979.tb02498.x.
- Jump up^ Pillans PI, Woods DJ (September 1995). “Adverse reactions associated with nefopam”. New Zealand Medical Journal. 108 (1008): 382–4. PMID 7566787.
- Jump up^ Sunshine A, Laska E (November 1975). “Nefopam and morphine in man”. Clinical Pharmacology and Therapeutics. 18 (5 Pt 1): 530–4. PMID 1102231.
- Jump up^ Phillips G, Vickers MD (October 1979). “Nefopam in postoperative pain”. British Journal of Anaesthesia. 51 (10): 961–5. PMID 391253. doi:10.1093/bja/51.10.961.
- ^ Jump up to:a b Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS (1980). “Nefopam: a review of its pharmacological properties and therapeutic efficacy”. Drugs. 19 (4): 249–67. PMID 6991238. doi:10.2165/00003495-198019040-00001.
- Jump up^ Tigerstedt I, Tammisto T, Leander P (December 1979). “Comparison of the analgesic dose-effect relationships of nefopam and oxycodone in postoperative pain”. Acta Anaesthesiologica Scandinavica. 23 (6): 555–60. PMID 397711. doi:10.1111/j.1399-6576.1979.tb01486.x.
- Jump up^ Gasser JC, Bellville JW (August 1975). “Respiratory effects of nefopam”. Clinical Pharmacology and Therapeutics. 18 (2): 175–9. PMID 1097153.
- Jump up^ Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M (January 2005). “Nefopam and Ketamine Comparably Enhance Postoperative Analgesia”. Anesthesia and Analgesia. 100 (1): 169–74. PMC 1283103
 . PMID 15616073. doi:10.1213/01.ANE.0000138037.19757.ED.
. PMID 15616073. doi:10.1213/01.ANE.0000138037.19757.ED. - Jump up^ Bilotta, F; Rosa, G (December 2000). “Nefopam for severe hiccups.”. The New England Journal of Medicine. 343 (26): 1973–4. PMID 11186682. doi:10.1056/nejm200012283432619.
- ^ Jump up to:a b c d e f g “Data Sheet ACUPAN™ Nefopam hydrochloride 30 mg tablets 20 mg intramuscular injection” (PDF). Medsafe New Zealand. iNova Pharmaceuticals (New Zealand) Limited. 3 September 2007. Retrieved 10 March 2014.
- ^ Jump up to:a b Bismuth, C; Fournier, PE; Bavoux, E; Husson, O; Lafon, D (September 1987). “[Chronic abuse of the analgesic nefopam (Acupan)].”. Journal de Toxicologie Clinique et Experimentale (in French). 7 (5): 343–6. PMID 3448182.
- ^ Jump up to:a b Tracqui, A; Berthelon, L; Ludes, B (May 2002). “Fatal overdosage with nefopam (Acupan).” (PDF). Journal of Analytical Toxicology. 26 (4): 239–43. PMID 12054367. doi:10.1093/jat/26.4.239.
- Jump up^ Roth, BL; Driscol, J. “PDSP Ki Database”. Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ Jump up to:a b Gregori-Puigjané, E.; Setola, V; Hert, J; Crews, BA; Irwin, JJ; Lounkine, E; Marnett, L; Roth, BL; Shoichet, BK (18 June 2012). “Identifying mechanism-of-action targets for drugs and probes” (PDF). Proceedings of the National Academy of Sciences. 109 (28): 11178–11183. PMC 3396511
 . PMID 22711801. doi:10.1073/pnas.1204524109.
. PMID 22711801. doi:10.1073/pnas.1204524109. - Jump up^ Bausch & Lomb (NZ) Ltd (17 May 2017). “NEW ZEALAND DATA SHEET ACUPAN(TM)” (PDF). Medsafe. New Zealand The Ministry of Health. Retrieved 4 September 2017.
- Jump up^ Kim, KH; Abdi, S (April 2014). “Rediscovery of nefopam for the treatment of neuropathic pain.”. The Korean Journal of Pain. 27 (2): 103–11. PMC 3990817
 . PMID 24748937. doi:10.3344/kjp.2014.27.2.103.
. PMID 24748937. doi:10.3344/kjp.2014.27.2.103. - ^ Jump up to:a b Camille Georges Wermuth; David Aldous; Pierre Raboisson; Didier Rognan (1 July 2015). The Practice of Medicinal Chemistry. Elsevier Science. pp. 250–251. ISBN 978-0-12-417213-5.
- Jump up^ Walter Sneader (23 June 2005). Drug Discovery: A History. John Wiley & Sons. pp. 405–. ISBN 978-0-471-89979-2.
- Jump up^ Hugo Kubinyi; Gerhard MÃ1⁄4ller (6 March 2006). Chemogenomics in Drug Discovery: A Medicinal Chemistry Perspective. John Wiley & Sons. pp. 54–. ISBN 978-3-527-60402-9.
- Jump up^ Amy Cruz (2014). Therapeutic Hypothermia. CRC Press. pp. 176–. GGKEY:R0AP2X4GZYF.
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Acupan |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral, intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low[1] |
| Protein binding | 70–75% (mean 73%)[1][2] |
| Metabolism | Liver (N–demethylation, others)[1] |
| Metabolites | Desmethylnefopam, others[1] |
| Biological half-life | Nefopam: 3–8 hours[1] Desmethylnefopam: 10–15 hours[1] |
| Excretion | Urine: 79.3%[1] Feces: 13.4%[1] |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ECHA InfoCard | 100.033.757 |
| Chemical and physical data | |
| Formula | C17H19NO |
| Molar mass | 253.34 g/mol |
| 3D model (JSmol) | |
////////////Nefopam Hydrochloride, Fenazoxine, Нефопама Гидрохлорид, 塩酸ネホパム
CN1CCOC(C2=CC=CC=C2C1)C3=CC=CC=C3


















