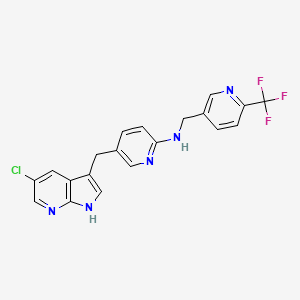
Pexidartinib
PLX-3397
5-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-N-((6-(trifluoromethyl)pyridin-3-yl)methyl)pyridin-2-amine
N-[5-[(5-Chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-2-pyridinyl]-6-(trifluoromethyl)-3-pyridinemethanamine
5-[(5-Chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-N-[[6-(trifluoromethyl)pyridin-3-yl]methyl]pyridin-2-amine hydrochloride
Phase III
A Multi-targeted tyrosine kinase inhibitor potentially for the treatment of tenosynovial giant cell tumor (TGCT).
![]() CAS 1029044-16-3
CAS 1029044-16-3
C20H15ClF3N5, 417.81
Pexidartinib; 1029044-16-3; PLX-3397; 5-((5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl)-N-((6-(trifluoromethyl)pyridin-3-yl)methyl)pyridin-2-amine; 5-[(5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl)methyl]-N-[[6-(trifluoromethyl)pyridin-3-yl]methyl]pyridin-2-amine; 5-[(5-Chloro-1h-Pyrrolo[2,3-B]pyridin-3-Yl)methyl]-N-{[6-(Trifluoromethyl)pyridin-3-Yl]methyl}pyridin-2-Amine;
- Originator Plexxikon
- Developer Barbara Ann Karmanos Cancer Institute; Columbia University; Merck & Co; National Cancer Institute (USA); Plexxikon; University of California at San Francisco
- Class 2 ring heterocyclic compounds; Antineoplastics; Fluorine compounds; Pyridines; Pyrroles; Small molecules
- Mechanism of Action Fms-like tyrosine kinase 3 inhibitors; Immunomodulators; Macrophage colony stimulating factor receptor antagonists; Proto oncogene protein c-akt inhibitors; Proto oncogene protein c-kit inhibitors
- Orphan Drug Status Yes – Giant cell tumour of tendon sheath; Pigmented villonodular synovitis
- Phase III Pigmented villonodular synovitis
- Phase II Glioblastoma; Malignant melanoma; Prostate cancer
- Phase I/II Breast cancer; Leukaemia; Peripheral nervous system diseases; Sarcoma; Solid tumours
- Phase I Gastrointestinal stromal tumours
- No development reported Neurological disorders; Rheumatoid arthritis
- Discontinued Acute myeloid leukaemia; Hodgkin’s disease
Most Recent Events
- 25 May 2016 Plexxikon and AstraZeneca plan the MEDIPLEX phase I trial for Solid tumours (Combination therapy, Metastatic disease) in France (NCT02777710)
- 05 Apr 2016 Daiichi Sankyo plans a phase I trial for Solid tumours (Late-stage disease, Second-line therapy or greater) in Taiwan (PO) (NCT02734433)
- 11 Mar 2016 Plexxikon re-initiates enrolment in a phase Ib trial in Solid tumours and Gastrointestinal stromal tumours in USA (NCT02401815)
Plexxikon, a subsidiary of Daiichi Sankyo, is developing pexidartinib, a dual Fms/Kit and FIt3-ITD inhibitor, for treating cancer.


Multi-targeted receptor tyrosine kinase inhibitor of CSF1R, c-Kit, and FLT3 (IC50 values 13 nM, 27 nM, and 11 nM, respectively) Administration of PLX3397 reduced CIBP, induced substantial intratumoral fibrosis, and was also highly efficacious in reducing tumor cell growth, formation of new tumor colonies in bone, and pathological tumor-induced bone remodeling. PLX3397 is superior to imatinib in the treatment of malignant peripheral nerve sheath tumor (MPNST), and the combination of PLX3397 with a TORC1 inhibitor could provide a new therapeutic approach for the treatment of this disease.
Plexxikon is conducting phase III clinical studies with PLX-3397 for the treatment of pigmented villonodular synovitis. Phase II clinical studies are ongoing for the oral treatment of melanoma and glioblastoma multiforme. Additional early clinical trials are underway for the treatment of metastatic breast cancer, for the treatment of prostate cancer (adenocarcinoma), and for the treatment of malignant peripheral nerve sheath tumor. No recent development has been reported from preclinical studies for the treatment of systemic lupus erythematosus and for the treatment of multiple sclerosis. Prior to patient enrollment, a phase I clinical trial by Plexxikon for the treatment of rheumatoid arthritis was withdrawn. Daiichi Sankyo (parent of Plexxikon) decided to discontinue phase II trials of the product for the treatment of castration-resistant prostate cancer and for the treatment of Hodgkin’s lymphoma after reviewing its clinical study results and also have discontinued phase II studies for the treatment of acute myeloid leukemia due to strategic reasons.
In 2014, orphan drug designation was assigned to the compound in the US for the treatment of pigmented villonodular synovitis andf giant cell tumor of the tendon sheath. In 2015, the compound was granted orphan designation in the E.U. for the treatment of tenosynovial giant cell tumor, localised and diffuse type. In the same year, the product was granted breakthrough therapy designation for the treatment of tenosynovial giant cell tumor (TGCT) where surgical removal of the tumor would be associated with potentially worsening functional limitation or severe morbidity.
C-fms and c-kit arc both type III transmembrane receptor protein tyrosine kinases (RPTKs) that regulate key signal transduction cascades that control cellular growth and proliferation. Both receptors have similar structural features comprising five extracellular immunoglobulin (IG) domains, a single transmembrane domain, and a split cytoplasmic kinase domain separated by a kinase insert segment.
c-Fms
C-fms is a member of the family of genes originally isolated from the Susan McDonough strain ot teline sarcoma viruses, The cellular proto-oncogene FMS (c-fms, cellular feline McDonough sarcoma) codes for the receptor for the macrophage colony-stimuktmg tactor (M- CSF) C-fms is crucial for the growth and differentiation of the monocyte-macrophage lineage, and upon binding of Vf-CSF to the extracellular domain of c-fms, the receptor dimeπzes and trans- autophosphorylates cytoplasmic tyrosine residues
M-CSF, first described by Robinson and co-workers (Blood 1969, 33 396-9), is a cytokine that controls the production, differentiation, and function of macrophages M-CSF stimulates differentiation of progenitor cells to mature monocytes, and prolongs the survival of monocytes Furthermore, M-CSF enhances cytotoxicity, superoxide production, phagocytosis, chemota\is, and secondary cytokine production of additional factors in monocytes and macrophages Examples of such additional factors include granulocyte colony stimulating lactor (G-CSF) interleukin-6 (IL-6), and mterleukm-8 (IL-8) M-CSF stimulates hematopoiesis, promotes differentiation and proliferation of osteoclast progenitor cells, and has profound effects on lipid metabolism Furthermore, M-CSF is important in pregnancy Physiologically, large amounts of M-CSF are produced in the placenta, and M-CSF is believed to play an essential role in trophoblast differentiation (Motoyoshi, lnt J Hematol 1998, 67 109-22) l hc elevated semm levels of M-CSF m early pregnancy may participate in the immunologic mechanisms responsible for the maintenance of the pregnancy (Flanagan & Lader, Curr Opm Hematol 1998, 5 181-5)
Related to c-fms and c-kit are two p_latelet -derived growth factor receptors, alpha (i e , pdgfra) and beta (pdgfrb) (PDGF) 1 he gene coding for pdgfra is located on chromosome 4ql 1 -q!2 in the same region of chromosome 4 as the oncogene coding for c-kit The genes coding for pdgfra and c-fms appear to have evolved from a common ancestral gene by gene duplication, inasmuch as these two genes are tandemly linked on chromosome 5 They are oriented head to tail with the 5-pnme exon of the c-fms gene located only 500 bp from the last 3-pπme exon of the gene coding for pdgfra Most gastrointestinal stromal tumors (GIST) have activating mutations in c-kit and most patients with GISTs respond well to Gleevec, which inhibits c-kit Hemπch et al (Science 2003, 299 “OS-IO) have shown that approximately 35% of GISTs lacking c-krt mutations, have intragenic activation mutations m tht gene encoding pdgfra, and that tumors expressing c-kit or pdgfrd are indistinguishable with respect to activation of downstream signaling intermediates and cytogenetic changes associated with tumor progression Thus, c kit and pdgfra mutations appear to be alternative and mutually exclusive oncogenic mechanisms m GISTs [0007} Similarly, the observation that production of M-CSF, the major macrophage growth factor, is increased in tissues during inflammation points out a role for c-frns in diseases, such as for example inflammatory diseases. More particularly, because elevated levels of M-CSF are found in the disease state, modulation of the activity of c-fms can ameliorate disease associated with increased levels of M-CSF.
c-Kit
The Stem Cell Factor (SCF) receptor c-kit plays an important role in the development of melanocytes and mast, germ and hematopoietic cells. Stem Cell Factor (SCF) is a protein encoded by the Sl locus, and has also been called “kit ligand” (KL) and mast cell growth factor (MGF), based on the biological properties used to identify it (reviewed in Tsujimura, Pathol Int 1996, 46:933-938; Loveland, et al., J. Endocrinol 1997, 153:337-344; Vliagoftis, et al,, Clin Immunol 1997, 100:435-440; Broudy, Blood 1997, 90: 1345-1364; Pignon, Hermatol Cell Ther 1997, 39: 1 14-1 16; and Lyman, et al., Blood 1998, 91 : 1 101 -1 134.). Herein the abbreviation SCF refers to the physiological ligand for c-kit.
SCF is synthesized as a transmembrane protein with a molecular weight of 220 or 248 Dalton, depending on alternative splicing of the mRNA to encode exon 6. The larger protein can be proteolytically cleaved to form, a soluble, glycosylated protein which noncovalently dimerizcs. Both the soluble and membrane-bound forms of SCF can bind to and activate c-kit. For example, in the skin, SCF is predominantly expressed by fibroblasts, keratinocytes, and endothelial cells, which modulate the activity of melanocytes and mast cells expressing c-kit. In bone, marrow stromal cells express SCF and regulate hematopoiesis of c-kit expressing stem cells. In the gastrointestinal tract, intestinal epithelial cells express SCF and affect the interstitial cells of Cajal and intraepithelial lymphocytes. In the testis, Sertoli cells and granulosa cells express SCF which regulates spermatogenesis by interaction with c-kit on germ cells.
PATENT
PATENT
WO 2008064265
PATENT
WO 2008064255
PATENT
Fragments in the clinic: PLX3397
Practical Fragments covers a wide variety of journals. J. Med. Chem., Bioorg. Med. Chem. Lett., Drug Disc. Today, and ACS Med. Chem. Lett. are all well-represented, but we also range further afield, from biggies such asNature and Science to more niche titles such as ChemMedChem, Acta. Cryst. D., and Anal. Chim. Acta. The increasingly clinical relevance of fragment-based approaches is highlighted by a recent paper by William Tap and a large group of collaborators appearing in the New England Journal of Medicine. This reports on the results of the Daiichi Sankyo (née Plexxikon) drug PLX3397 in a phase I trial for tenosynovial giant-cell tumor, a rare but aggressive cancer of the tendon sheath.
The story actually starts with a 2013 paper by Chao Zhang and his Plexxikon colleagues in Proc. Nat. Acad. Sci. USA. The researchers were interested in inhibiting the enzymes CSF1R (or FMS) and KIT; both kinases are implicated in cancer as well as inflammatory diseases. The team started with 7-azaindole, the same fragment they used to discover vemurafenib. Structural studies of an early derivative, PLX070, revealed a hydrogen bond between the ligand oxygen and a conserved backbone amide. Further building led to PLX647, with good activity against both CSF1R and KIT. Selectivity profiling against a panel of 400 kinases revealed only two others with IC50values < 0.3 µM. The molecule was active in cell-based assays, had good pharmacokinetics in mice and rats, and was active in rodent models of inflammatory disease.
The new paper focuses on the results of a clinical trial with PLX3397, a derivative of PLX647. Despite its close structural similarity to PLX647, it binds to CSF1R in a slightly different manner. Both inhibitors bind to the inactive form of the kinase, but PLX3397 also recruits the so-called juxtamembrane domain of the kinase to stabilize this autoinhibited conformation. Pharmacokinetic and pharmacodynamics studies in animals were also positive.

http://practicalfragments.blogspot.in/2015/10/fragments-in-clinic-plx3397.html
Tenosynovial giant-cell tumor seems to be dependent on CSF1R, so the researchers performed a phase 1 dose-escalation study with an extension in which patients treated with the chosen phase 2 dose were treated longer. Of the 23 patients in this extension, 12 had a partial response and 7 had stable disease. A quick search ofclinicaltrials.gov reveals that PLX3397 is currently in multiple trials for several indications, including a phase 3 trial for giant cell tumor of the tendon sheath.
Several lessons can be drawn from these studies. First, as the authors note, one fragment can give rise to multiple different clinical candidates. Indeed, in addition to vemurafenib, 7-azaindole was also the starting point forAZD5363. This is a good counterargument to those who believe that novelty is essential in fragments.
A second, related point is that selectivity is also not necessary for a fragment. The fact that 7-azaindole comes up so frequently as a kinase-binding fragment has not prevented researchers from growing it into remarkably selective inhibitors. An obvious corollary is that even subtle changes to a molecule can have dramatic effects: the added pyridyl nitrogen in PLX3397 is essential for stabilizing a unique conformation of the enzyme.
NEW PATENT
The compound named, [5-(5-chloro-lH-pyrrolo[2,3-b]pyridin-3-ylmethyl)-pyridin-2-yl]-(6-trifluoromethyl-pyridin-3-ylmethyl)-amine, which is also known as pexidartinib, is effective for treating subjects suffering from or at risk of a c-Kit and/or c-Fms and/or Flt3 mediated disease or condition. Suitable compounds, including pexidartinib or a salt thereof, for the treatment of such diseases and conditions are disclosed in U.S. Patent 7,893,075, U.S.
Publication No. 2014-0037617 and U.S. Publication No. 2013-0274259, the disclosures of all of which are incorporated herein by reference in their entirety.
There remains a need in developing new versatile and facile processes for the efficient preparation of pexidartinib and other similar molecules, especially in an industrial scale.
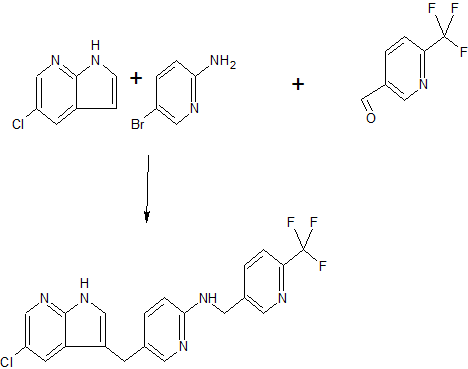
Example 1. Synthesis of [5-(5-chloro-lH-pyrrolo[2,3-b]pyridin-3-ylmethyl)-pyridin-2-yl]- (6-trifluoromethyl-pyridin-3-ylmethyl)-amine:

Step 1: Conversion of A to Ilia
The reactor was charged with Compound A (1000 gm, 1.0 eq.), Compound B (497 gm, 1.05 eq.), tetrabutylammonium hydrogen sulfate (31.6 gm, 0.03 eq.) and isopropanol (12 L, 11.8 vol). The reaction mixture was stirred for at least about an hour to obtain a near clear, yellow solution. Then potassium ie/ -pentoxide (73 mL, 0.04 eq.) was added over 30 seconds. The reaction mixture was stirred at about 15-25°C for about 20-24 hours. The reaction was monitored by HPLC. When the content of compound Ilia was more than 80%, the reaction was deemed complete. The reaction mixture was cooled to about 0-10°C and then stirred for at least about 2 hours. The precipitate was filtered, washed with 3 L isopropanol that had been cooled to 0°C and dried to provide compound Ilia as a white solid (1.34 kg, 91.2% yield, 97.7% purity by HPLC). 1H NMR (DMSO-d6): δ (ppm) 11.8 (s, NH), 8.50-8.51 (d, 1H), 8.17 (d, 1H), 7.85-7.88 (dd, 1H), 7.82 (d, 1H), 7.41 (S, 1H), 7.29-7.31 (d, 1H), 6.04 (s, 2H), and 1.35 (s, 18H).
[0073] Alternatively, potassium ie/ -pentoxide can also be used in this reaction as a 25% solution in toluene.
Step 2: Conversion of Ilia to IV
The reactor was charged with compound Ilia (1.1 kg, 1 eq.) and acetonitrile (8.8 L, 12.4 vol) and the reaction mixture was stirred. Then triethylsilane (1.35 kg, 5 eq.) was added at about
15-30°C over at least about 10 minutes. Then trifluoroacetic acid (2.38 kg, 9 eq.) was added to the reactor at about 15-30°C over at least about 30 minutes. The reaction mixture was heated at about 55-65°C over at least about 4 hours. It was then stirred at about 55-65°C for about 20-48 hours. The reaction was monitored by HPLC. When the content of compound Ilia was less than about 1%, the reaction was deemed complete. The reaction mixture was cooled to about 45-55°C and then a) concentrated to 3.3 L under vacuum and b) water (8.25 L) was charged. Steps a) and b) were repeated 4 times. The reaction mixture was then heated at about 45-60°C and stirred for bout 1-3 hours. It was then cooled to about 0-10°C over at least about 2 hours and it was stirred at about 0-10°C for about 2-4 hours. The precipitate was filtered, washed with 2.2 L water and then with heptane (1.1 L) and dried to provide the TFA salt of compound IV as an off-white solid (673.3 gm, 77.9% yield, 99.7% purity by HPLC). 1H NMR (DMSO-d6): δ (ppm) 11.78 (s, COOH), 8.18 (d, 1H), 8.08-8.09 (broad doublet, 2H), 7.93-7.94 (d, 1H), 7.81-7.84 (dd, 1H), 7.47-7.48 (d, 1H), 6.90-6.93 (d, 1H), 3.92 (s, 2H).
Step 3: Conversion of IV to I
[0074] The reactor was charged with compound IV (663.3 gm, 1 eq.), compound V (623.2 gm, 2.0 eq.) and acetonitrile (13.3 L). The reaction mixture was stirred for about 5-10 minutes at room temperature. Triethylsilane (1531.6 gm, 7.4 eq.) was then added to the reactor over at least about 10 minutes at or less than about 30°C. Trifluoroacetic acid (1542.5 gm, 7.6 eq.) was added to the reactor over at least about 10 minutes at or less than about 30°C. The reaction mixture was stirred for at least about 30 minutes at about 15-30°C. It was then heated to about 70-82°C over at least about one hour and then stirred at about 70-82°C for about 20-48 hours. The reaction was monitored by HPLC. When the content of compound IV was less than about 1%, the reaction was deemed complete.
[0075] The reaction mixture was cooled to room temperature, the acetonitrile layer was separated and concentrated. Then water (7.96 L) was charged and the reaction mixture was concentrated to 6.64 L under vacuum providing a tri-phasic mixture. It was then cooled to 15- 25°C, charged with ethyl acetate (10.6 L) and stirred providing a biphasic mixture. It was cooled to 0-10°C, charged with a 25% NaOH solution in water until a pH of about 8-9 was reached with vigorous stirring, heated to about 65-75°C and stirred at about 65-75° for about 30 minutes. The organic layer was separated, and water (3.98 L) was charged and the reaction mixture was heated at about 65-75°C. The organic layer was separated and concentrated to about 5.3-5.9 L under vacuum, heptane (11.9 L) was added and the slurry was heated to about 55-65°C and stirred for about 2 hours. The reaction mixture was cooled to about 15-30°C over at least about 2 hours and then stirred at about 15-30°C for at least about 1 hour. The precipitate was filtered, washed with heptane (1.99 L) and dried. The filter cake was charged into reactor with ethyl acetate (5.31L, 8 vol) and heptane (2.65 L, 4 vol), cooled to about 15-30°C over at least about 2 hours and then stirred at about 15-30°C for at least about 1 hour. The precipitate was filtered, washed with heptane and dried to provide Compound I as a light yellow solid (648.4 gm, 89.4% yield, 99.4% purity by HPLC).
Step 4: Conversion of I to II
[0076] The reactor was charged with compound I (10 gm, 1 eq.), 110 mL ethanol was added and the reaction mixture was stirred. Concentrated hydrochloric acid (4.7 gm, 2 eq.) was slowly added to the reaction mixture while maintaining a temperature of about 30°C or less to form a clear solution. It was then filtered and washed with methanol (10 mL). It was again filtered and purified water (3 mL) was added to it at about 28-32°C. The mixture was stirred at about 28-32°C for 1-3 hours and filtered, purified water (177 mL) was added to it at about 25-32°C. The reaction mixture was cooled at about 0-7 °C and stirred for at least about 2 hours. Optionally, seed crystals of compound II can be added in this step. The solids were filtered, rinsed with acool (0-5°C) mixture of methanol (6 mL) and MTBE (24 mL), and with cool (0-5°C) MTBE (30 mL). The product was dried to provide Compound II (90% yield).
US20152655862015-09-24COMPOUNDS MODULATING C-FMS AND/OR C-KIT ACTIVITY AND USES THEREFOR
US20142433652014-08-28COMPOUNDS MODULATING C-FMS AND/OR C-KIT ACTIVITY AND USES THEREFOR
US87227022014-05-13Compounds modulating c-fms and/or c-kit activity and uses therefor
US20140458402014-02-13COMPOUNDS AND METHODS FOR KINASE MODULATION, AND INDICATIONS THEREFOR
US20132742592013-10-17KINASE MODULATION AND INDICATIONS THEREFOR
US84047002013-03-26Compounds modulating c-fms and/or c-kit activity and uses therefor
US20112304822011-09-22COMPOUNDS MODULATING C-FMS AND/OR C-KIT ACTIVITY
US78930752011-02-22Compounds modulating c-fms and/or c-kit activity and uses therefor
//////1029044-16-3, Pexidartinib , PLX-3397, PHASE3
FC(F)(F)c1ccc(cn1)CNc2ccc(cn2)Cc4cnc3ncc(Cl)cc34
 .
.