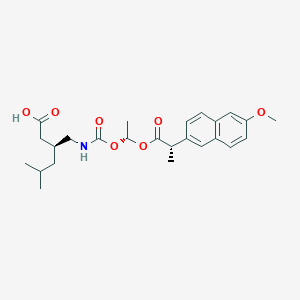
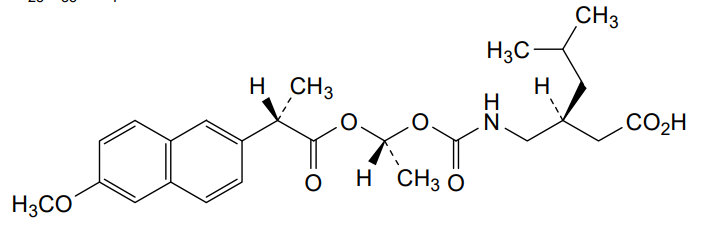
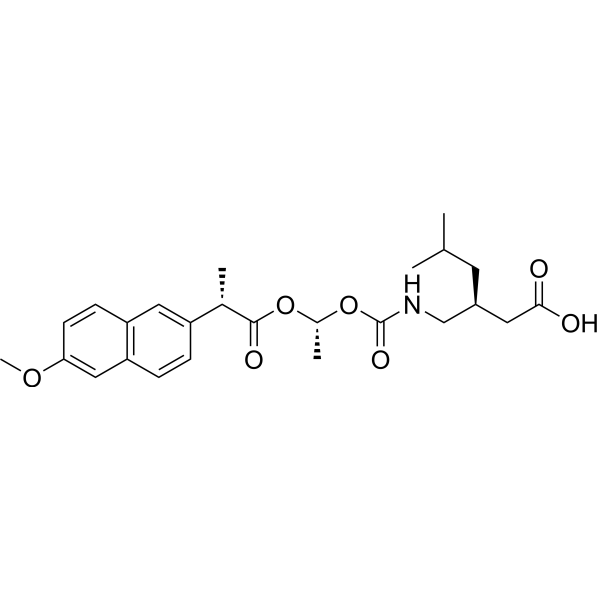
Pregabalin naproxencarbil
CAS 1221072-91-8
MF C25H33NO7 MW459.5 g/mol
(3S)-3-[({[(1R)-1-{[(2S)-2-(6-methoxynaphthalen-2-yl)propanoyl]oxy}ethoxy]carbonyl}amino)methyl]-5-
methylhexanoic acid
(3S)-3-[[[(1R)-1-[(2S)-2-(6-methoxynaphthalen-2-yl)propanoyl]oxyethoxy]carbonylamino]methyl]-5-methylhexanoic acid
gabamimetic, analgesic, ZVG8DDT3FJ
- OriginatorXgene Pharmaceutical
- ClassAminobutyric acids; Analgesics; Antiepileptic drugs; Antipyretics; Antirheumatics; Anxiolytics; Drug conjugates; Gabapentinoids; Naphthaleneacetic acids; Neuroprotectants; Nonsteroidal anti-inflammatories; Small molecules
- Mechanism of ActionCACNA2D1 protein modulators; Cyclooxygenase inhibitors
- Phase II/IIIPostoperative pain
- Phase IIAcute pain; Cancer pain; Pain
- Phase I/IIBack pain; Neuropathic pain
- No development reportedDiabetic neuropathies
- 15 Jul 2025XG005 licensed to NeuroGen in China, Hong Kong, and Macau
- 31 Dec 2024Efficacy and adverse events data from phase-II/III trial in Postoperative pain released by Xgene Pharmaceutical
- 25 Oct 2024Xgene Pharmaceutical completes the phase II/III trial in Postoperative pain in USA (PO)
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019219000&_cid=P22-MID0L3-49648-1
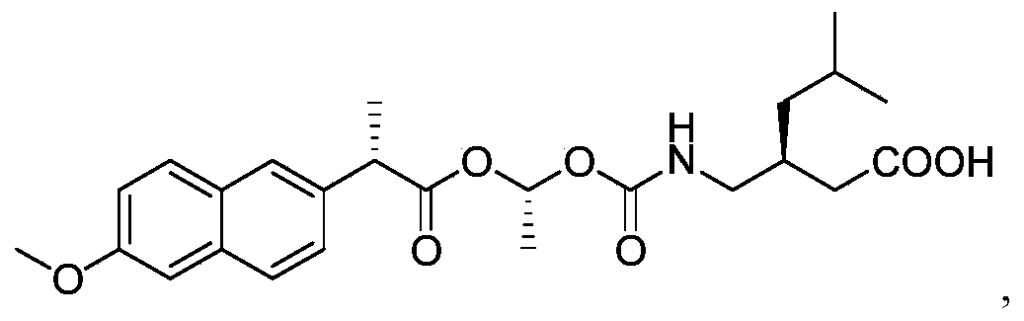
Example 1: Exemplary synthesis of the compound of Formula I and crystallization thereof
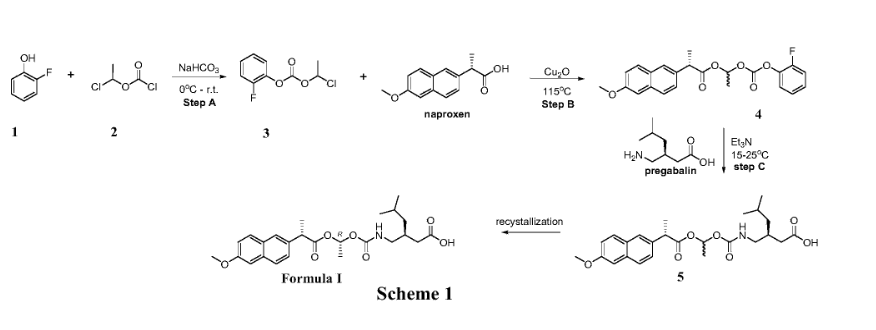
Step A: Synthesis of 1-chloroethyl 2-fluorophenyl carbonate (3)
[0306]
A suitable reaction vessel was charged with water and sodium bicarbonate followed by the starting material 2-fluoro-phenol (1) . The mixture was cooled to a temperature of about 0~5℃ and 1-chloroethyl chloroformate (2) was added slowly while maintaining the temperature at 0~5℃. The temperature was raised to about 15 ± 5℃. When the reaction was judged complete by the disappearance of 2-fluoro-phenol (criteria: ≤ 2.0%, by HPLC) the reaction was worked up. n-Heptane was added and the organic phase was separated, washed with water and brine. The solution was concentrated, then toluene was added and the solution was concentrated again. The toluene addition and the concentration cycle were repeated once more.
[0307]
Step B: Synthesis of (S) – ( (R, S) -1- ( (2-fluorophenoxy) carbonyloxy) ethyl 2- (6-methoxynaphthalen-2-yl) propanoate (4)
[0308]
To a solution of naproxen in toluene in a suitable reaction vessel, 1-chloroethyl 2-fluorophenyl carbonate (3) and cuprous oxide was added. The temperature of the mixture was raised to about 115 ±5℃. When the reaction was judged complete by the disappearance of 1-chloroethyl 2-fluorophenyl carbonate (criteria: ≤ 2.5%, by HPLC) the reaction was worked up. Methyl tert-butyl ether was added at about 50 ± 5℃. The resulting mixture was filtered and the filtrate was collected at about 25 ± 5℃. Purified water was added into the filtrate and then the mixture was cooled to about 0 ± 5℃. The mixture was alkalified with ammonium hydroxide to a pH of about 9~11 and the organic phase was separated and washed with ammonium hydroxide and brine. The solution was concentrated, then acetonitrile was added and the solution was concentrated again. The acetonitrile addition and the concentration cycle were repeated some more times until the residual toluene was not more than 10% (by GC method) .
[0309]
Step C: Synthesis of (S) -3- ( ( ( (R) -1- ( (S) -2- (6-methoxynaphthalen-2-yl) propanoyloxy) ethoxy) carbonyl-amino) methyl) -5-methylhexanoic acid (the compound of Formula I)
[0310]
To a solution of the mixture of (S) – ( (R, S) -1- ( (2-fluorophenoxy) carbonyloxy) ethyl 2- (6-methoxynaphthalen-2-yl) propanoate (4) in acetonitrile and methyl tert-butyl ether in a suitable reaction vessel, purified water and pregabalin was charged. Triethylamine was added slowly while maintaining the temperature at about 15 ± 5℃. The temperature was raised to about 25 ± 3℃ for reaction. When the reaction was judged complete by the disappearance of (S) – ( (R, S) -1- ( (2-fluorophenoxy) carbonyloxy) ethyl 2- (6-methoxynaphthalen-2-yl) propanoate (criteria: ≤ 0.5%, by HPLC) the reaction was worked up. The resulting mixture was acidified with KHSO 4to pH 3~5 and extracted with methyl tert-butyl ether. The combined organic layers were washed with purified water and brine. The organic phase was then concentrated. Isopropanol was added and the solution was concentrated again. The isopropanol addition and the concentration cycle was repeated once or more times until the total residual acetonitrile and methyl tert-butyl ether was not more than 5% (by GC method) . n-Heptane was added into the mixture at about 40~45 ℃ and stirred and then the temperature was gradually lowered at set appropriate intervals to crystallize (S) -3- ( ( ( (R) -1- ( (S) -2- (6-methoxynaphthalen-2-yl) propanoyloxy) ethoxy) carbonyl-amino) methyl) -5-methylhexanoic acid (5) from the system. When the precipitation was complete, the heterogeneous mixture was centrifuged and the solid was collected.
[0311]
The crude product of (S) -3- ( ( ( (R) -1- ( (S) -2- (6-methoxynaphthalen-2-yl) propanoyloxy) ethoxy) carbonyl-amino) methyl) -5-methylhexanoic acid (5) was added to a solution of isopropanol and water in a suitable vessel. The mixture was stirred while raising the temperature to 45 ± 3 ℃ until all the solid was dissolved, then the temperature was lowered gradually at set appropriate intervals to recrystallize (S) -3- ( ( ( (R) -1- ( (S) -2- (6-methoxynaphthalen-2-yl) propanoyloxy) ethoxy) carbonyl-amino) methyl) -5-methylhexanoic acid from the system. When the precipitation had stopped, the heterogeneous mixture was centrifuged and the expected pure (S) -3- ( ( ( (R) -1- ( (S) -2- (6-methoxynaphthalen-2-yl) propanoyloxy) ethoxy) carbonyl-amino) methyl) -5-methylhexanoic acid (Formula I) was collected.
Example 2: Alternative synthetic route of (S) -3- ( ( ( (R) -1- ( (S) -2- (6-methoxynaphthalen-2-yl) propanoyloxy) ethoxy) carbonyl-amino) methyl) -5-methylhexanoic acid
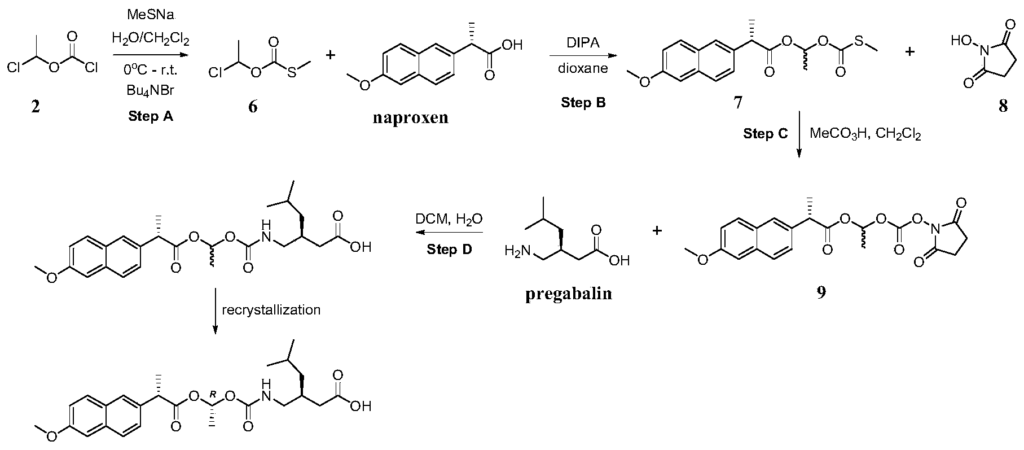
PAT
- Crystalline form of 1-(acyloxy)-alkyl carbamate drug complex of naproxen and pregabalinPublication Number: JP-7441181-B2Priority Date: 2018-05-14Grant Date: 2024-02-29
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: TW-I837128-BPriority Date: 2018-05-14Grant Date: 2024-04-01
- Method for preparing 1-(acyloxy)-alkyl carbamate drug complex of naproxen and pregabalin and intermediate thereofPublication Number: KR-102750784-B1Priority Date: 2018-05-14Grant Date: 2025-01-06
- GABA conjugates and methods of use thereofPublication Number: US-9186341-B2Priority Date: 2008-10-08Grant Date: 2015-11-17
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: US-2021221768-A1Priority Date: 2018-05-14
- Crystalline morphology of the 1- (acyloxy) -alkylcarbamate drug complex of naproxen and pregabalinPublication Number: JP-2021530434-APriority Date: 2018-05-14
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: AU-2019271799-B2Priority Date: 2018-05-14Grant Date: 2023-10-12
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: EP-4227293-A2Priority Date: 2018-05-14
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: EP-4227293-A3Priority Date: 2018-05-14
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: AU-2019271799-A1Priority Date: 2018-05-14
- crystalline forms of 1- (acyloxy) -alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: BR-112020022885-A2Priority Date: 2018-05-14
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: CN-112424158-APriority Date: 2018-05-14
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: EP-3793974-A1Priority Date: 2018-05-14
- Crystalline form of 1-(acyloxy)-alkyl carbamate drug complex of naproxen and pregabalinPublication Number: KR-20210013081-APriority Date: 2018-05-14
- Naproxen and pregabalin 1-(acyloxy)-alkyl carbamate drug conjugate purification processPublication Number: TW-202436285-APriority Date: 2022-11-24
- Naproxen and pregabalin 1- (acyloxy) -alkyl carbamate drug conjugate purification processPublication Number: WO-2024109817-A1Priority Date: 2022-11-24
- Crystalline forms of 1-(acyloxy)-alkyl carbamate drug conjugates of naproxen and pregabalinPublication Number: WO-2019219000-A1Priority Date: 2018-05-14
- Process for making 1-(acyloxy)-alkyl-carabmate drug conjugates of naproxen and pregabalinPublication Number: CA-3099775-A1Priority Date: 2018-05-14
- Naproxen (NAPROXEN) and pregabalin (PREGABALIN) 1-(acetyl)-alkylcarbamate drug conjugate crystalline formPublication Number: TW-202016065-APriority Date: 2018-05-14



AS ON OCT2025 4.511 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
.//////////Pregabalin naproxencarbil, gabamimetic, analgesic, ZVG8DDT3FJ














