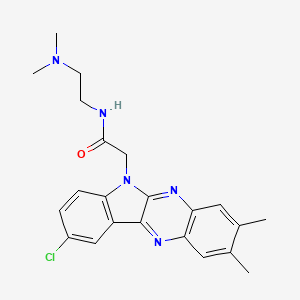
Rabeximod, ROB 803
C22H24ClN5O, 409.92
2-(9-chloro-2,3-dimethylindolo[3,2-b]quinoxalin-6-yl)-N-[2-(dimethylamino)ethyl]acetamide
CAS 872178-65-9
- OriginatorOxyPharma
- DeveloperCyxone; University of California
- ClassAcetamides; Anti-inflammatories; Disease-modifying antirheumatics; Heterocyclic compounds with 4 or more rings; Small molecules
- Mechanism of ActionCell differentiation modulators; Macrophage inhibitors
- Phase IICOVID 2019 infections; Rheumatoid arthritis
- 12 Oct 2021Cyxone terminates a phase-II trial in COVID-2019 infections in Slovakia (PO) (EudraCT2020-004571-41)
- 10 Aug 2021Cyxone completes a phase-II trial in COVID-2019 infections in Slovakia (PO) (EudraCT2020-004571-41)
- 23 Feb 2021Phase-II clinical trials in COVID-2019 infections in Slovakia (PO) (EudraCT2020-004571-41)
SYN
US 20050288296
https://patents.google.com/patent/US20050288296
SYN
WO 2014140321
https://patents.google.com/patent/WO2014140321A1/en
This compound was prepared as described in PCT/SE2005/000718 (WO 2005/123741), cf. “Compound E” at page 12 of said WO pamphlet.
Compound E
9-Chloro-2,3-dimerthyl-6-(N,N-dimethylaminoethylamino-2-oxoethyl)-6H-indolo- [2,3-b]quinoxaline (R1=Cl, R2=CH3, X=CO, Y=NH-CH2-CH2-R3; R3=NR5R6;
R5=R6=CH3)
Yield: 58%; 1H-NMR δ: 8.29 (d, 1H), 8.23 (t, 1H), 7.98 (s, 1H), 7.82 (s, 1H), 7.71
(dd, 1H), 7.61 (d, 1H), 5.09 (s, 2H), 3,16 (q, 2H), 2.47 (s, 6H), 2.28 (t, 2H), 2,12
(s, 6H);
SYN
Rabeximod is an orally administered compound for treatment of moderate or severe active rheumatoid arthritis that is currently undergoing phase II clinical testing in eight European countries.

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com
////////////////////////////////////////
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021250197&_cid=P11-KX9XFU-50498-1
The compound rabeximod has been described in European patent application publication EP1756111A1 later granted as EP1756111 B1. The preparation of rabex imod, as compound E, is specifically described in EP1756111A1 as a small-scale process without any description on how to develop a process that can be used for GMP and upscaled. Rabeximod was made in a 58% yield in a small-scale lab pro cess, but no parameters for scaling up have been disclosed.
The objective of the present invention is to provide a process that is suitable for large scale synthesis in good yield, with stable process parameters, and suitable for GMP production.
Experimental
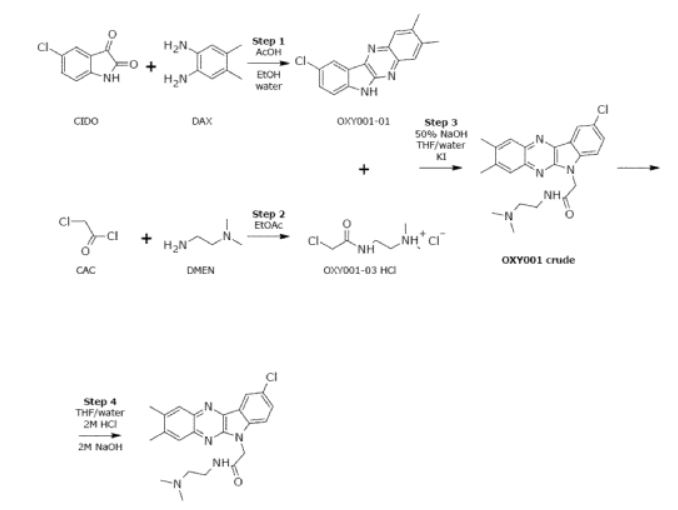
The current process to manufacture Rabeximod involves several process steps as illustrated in below reaction scheme and as described in detail hereunder.

Manufacturing Process OXY001-01 Intermediate
Starting materials: 5-Chloroisatin (CIDO) and 4,5-Dimethyl-1 ,2-phenylenediamine (DAX)
Table 1: Overview Required Raw Materials and Quantities Step 1
Table 2: Raw Materials Specifications Step 1
Resulting Product (Intermediate): OXY001-01
Batch size: 13.03 kg of OXY001-01
Process description: 4,5-Dimethyl-1 ,2-phenylenediamine (1.1 equivalent) was added to acetic acid (4.7 volumes) in reactor (reactor was running under nitrogen at atmospheric pressure) and stirred up to 3 hours at moderate rate at +20 to +25 °C until clear dark brown solution was formed. 4, 5-Dimethyl-1 ,2-phenylenediamine so lution in acetic acid solution was transferred to intermediate feeding vessel. 5-chloro-isatin (1 .0 equivalent) was added to acetic acid (14.3 volumes) in reactor and stirred while jacket temperature of reactor was adjusted to approximately +150 °C to achieve a reflux temperature for active reflux of solvents. When reflux temperature was reached the 4, 5-Dimethyl-1 ,2-phenylenediamine solution in acetic acid was slowly added over 2-3 hours while distilling acetic acid (4.7 volumes) from the reaction mix ture. A fresh portion of acetic acid (4.7 volumes) was added to the reactor at about the same rate as distillation (4.7 volumes) occurred. After distillation the reaction mixture was stirred at reflux temperature for at least another 2 hours. The expected appearance of content in the reactor was a dark yellow to orange slurry. The reaction mixture was cooled to +65 to +70 °C and filtered using a Nutsche filter using Polyes ter filter cloth (27 pm) or similar as filter media. The filter cake was washed 3 times with fresh ethanol (3 x 4.2 volumes) and 1 time with water (1 x 4.2 volume). After washing the filter cake was dried at +40 to +45 °C for 12 hours and additionally in a vacuum tray dryer for 12 hours at +40 °C resulting in a yellow to orange/brown solid. An in-process control sample was taken and analysed for loss on drying (LOD). LOD should be < 2% (w/w). If the LOD is > 2%, the vacuum tray dryer step was repeated.
Theoretical yield: 18.62 kg
Yield: 70±5% (13.03±0.96kg)
Maximum volume: 216 L
Manufacturing Process OXY001-03 HCI Intermediate
CAC DMEN OXY001-03 HCI
Starting materials: Chloroacetyl chloride (CAC) and N,N-Dimethylethylene diamine (DMEN)
Table 3: Overview Required Raw Materials and Quantities Step 2
a) mol//mol of DMEN; b) kg/kg of DMEN; c) L/kg of DMEN
Table 4: Raw Materials Specifications Step 2
Resulting Product (Intermediate): OXY001-03 HCI
Batch size: 22.6 kg of OXY001 -03 HCI
Process description: Chloroacetyl chloride (1.03 equivalents) was dissolved in ethyl acetate (15 volumes) in reactor (reactor was running under nitrogen at atmos pheric pressure) at +20 °C. The solution was stirred and cooled down to +10 °C.
N,N-dimethylethylene diamine (1.00 equivalent) solution in ethyl acetate (1.0 volume) was slowly charged to the reactor when the temperature reached a range from +10 to +25 °C and at such a rate over 1-2 hours that the internal temperature did not exceed +25 °C. The slurry was stirred for 5 to 30 minutes at +20 to +25 °C and filtered using a Nutch filter using Polyamide filter cloth (25 pm) or similar as filter media. The product was washed 3 times on the filter with ethyl acetate (3 x 5 volumes) and dried on the filter for at least 16 hours and additionally in a vacuum tray dryer for 12 hours at +40 °C resulting in an off-white to beige solid.
Theoretical yield: 25.09 kg
Yield: 90±5% (22.6±1 .25 kg)
Maximum volume: 202 L
Manufacturing Process OXY001 Crude
– OXY001 Crude Starting materials: OXY001-01 and OXY001-03 HCI
Table 5: Overview Required Raw Materials and Quantities Step 3
Table 6: Raw/Intermediate Materials Specifications Step 3
Resulting Product: OXY001 Crude (crude rabeximod) Batch size: 11.38 kg of OXY001 Crude
Process description: OXY001-01 (1.0 equivalent) was dissolved in tetrahydrofuran (15.4 volumes) and 50% NaOH aqueous solution (8.0 equivalents in relation to OXY001 -01 ) in reactor (reactor was running under nitrogen at atmospheric pressure) and mixed at +55 to +60 °C up to approximately 1 hour until clear dark red solution was formed. Potassium iodide (0.81 equivalents) was added under vigorous stirring and mixed for 10 to 30 minutes at +55 to +60 °C. OXY001-03 HCI (2.0 equivalents) was added to the solution and mixed for at least 2 hours at +55 to +60 °C. Following completion of the reaction, the mixture was quenched with water (15.4 volumes) and tetrahydrofuran removed (15.4 volumes) by evaporation under reduced pressure. The slurry was cooled to +20 to +25 °C and stirred for 1 hour and filtered with a Nutch filter using Polyamide filter cloth (25 pm) or similar as filter media. Resulting cake was washed 3 times with water (3 x 5 volumes) until the pH of the filtrate was between 8-7 and dried on the filter at +40 to +45 °C for at least 12 hours by air suction and additionally in a vacuum tray dryer for 12 hours at +40 °C. Afterwards resulting ma terial was suspended in in tetrahydrofuran (25 volumes) at +45 to +50 °C for at least 1 hour. OXY001 Crude was isolated by filtration with a Nutch filter using Polyamide filter cloth (25 pm) or similar as filter media and washed 2 times on the filter with tetrahydrofuran (2 x 7 volumes). Resulting cake was dried on the filter at +40 to +45 °C for at least 12 hours and additionally in a vacuum tray dryer for 12 hours at +40 °C.
Theoretical yield: 18.96 kg
Yield: 60±5% (11 38±0.95 kg)
Maximum volume: 500 L
Purification of crude Rabeximod:
OXY001 crude (1 .0 equivalent) was dissolved in tetrahydrofuran (10 volumes), water (3 volume), and 2M HCI (1.4 volumes) mixture. The solution was clear filtered and heated to +50 °C. pH of mixture was adjusted to 10-12 by addition of 2M NaOH (1.3 volume). The formed slurry was cooled to +20 to +25 °C and diluted with water (12 volumes).
After stirring for at least 12 hours the slurry was filtered at +20 to +25 °C and washed on the filter with tetrahydrofuran:water (5:2) mixture (2×3 volumes). Rabeximod has a molecular weight of 409.92 g/mol and is isolated as a crystalline free base having a melting point of 259-261 °C.
Batch release results of batches used in Phase 2 and Phase 1 clinical studies are provided in Table 7.
Purity is equal to or above 98% as measured by HPLC.
Table 7: Batch release results of Rabeximod drug substance batches used in Phase 1 and phase 2 clinical studies
/////////////////Rabeximod, ROB 803, UNII-J4D3K58W3Z, рабексимод , رابيكسيمود 雷贝莫德 ,OXYPHARMA, PHASE 2, CYXONE
CC1=CC2=C(C=C1C)N=C3C(=N2)C4=C(N3CC(=O)NCCN(C)C)C=CC(=C4)Cl