Regioselective access of alkylidendibenzo[c,f]oxocine framework via cyclocarbopalladation/cross-coupling cascade reactions and reductive Heck strategy
New J. Chem., 2017, Advance Article
DOI: 10.1039/C6NJ03825E, Paper
DOI: 10.1039/C6NJ03825E, Paper
a
Institute of Organic Chemistry, University of Würzburg, Am Hubland, 97074 Würzburg, Germany
E-mail: tapas.ghosh@uni-wuerzburg.de,tapasghosh.chem@gmail.com
Tel: +49-931-31-81629
E-mail: tapas.ghosh@uni-wuerzburg.de,tapasghosh.chem@gmail.com
Tel: +49-931-31-81629
New J. Chem., 2017, Advance Article
DOI: 10.1039/C6NJ03825E
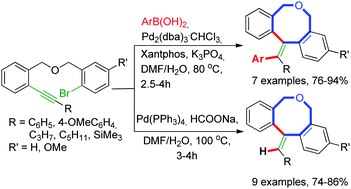
Palladium-catalyzed dual strategies of cascade cyclocarbopalladation/cross-coupling of alkynes and a reductive Heck reaction have been developed to construct dibenzo[c,f]oxocine frameworks with tri- and tetra-substituted exo-cyclic alkenes with high stereo- and regio-control.
Palladium-catalyzed dual strategies of cascade cyclocarbopalladation/cross-coupling of alkynes and a reductive Heck reaction have been developed to construct dibenzo[c,f]oxocine frameworks with tri- and tetra-substituted exo-cyclic alkenes with high stereo- and regio-control. The success of this efficient methodology has been demonstrated by the synthesis of a number of dibenzoxocines in moderate to good yields and in sufficient quantities to support their further development.
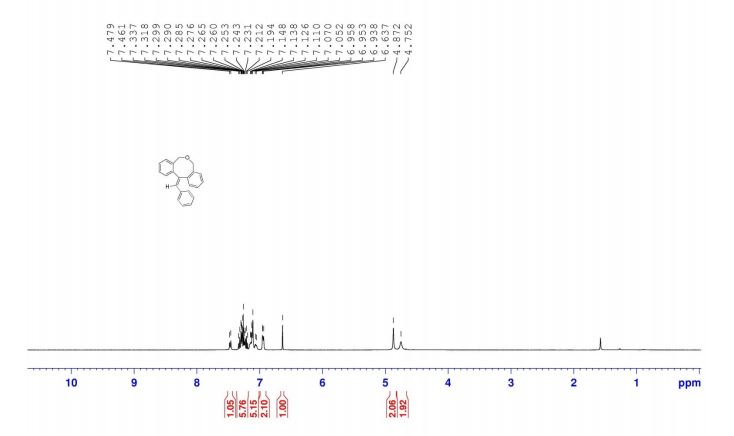
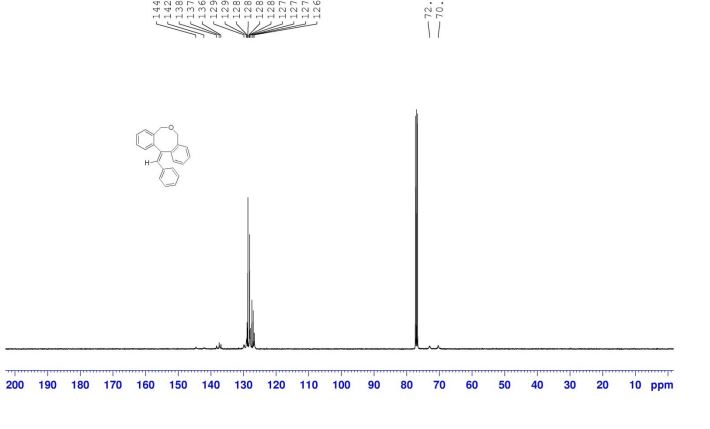
12-benzylidene-7,12-dihydro-5H-dibenzo[c,f]oxocine (8a): The material obtained after workup was subjected to column chromatography on silica gel with petroleum ether/EtOAc (19:1) as eluent to deliver pure 8a. Off white solid, yield = 82%,
mp. 134-136 oC,
IR (KBr): 2861, 1623, 1602 cm-1 , 1H NMR (400 MHz, CDCl3): δH = 7.47 (d, 1H, J = 7.2 Hz), 7.19-7.34 (m, 5H), 7.05-7.15 (m, 5H), 6.94-6.96 (m, 2H), 6.64 (s, 1H), 4.87 (s, 2H), 4.75 (s, 2H).
13C NMR (100 MHz, CDCl3): δC = 144.6, 142.1, 138.3, 137.4, 136.9, 129.9, 129.1, 128.9, 128.6, 128.3, 128.1, 127.9, 127.4, 127.0, 126.7, 73.0, 70.3.
HRMS (ESI [M+Na]+ ): for C22H18O calcd 321.1255; found 321.1245.
///////Regioselective, alkylidendibenzo[c,f]oxocine, cyclocarbopalladation/cross-coupling cascade reactions, reductive Heck strategy














