- Molecular FormulaC22H23FN2O2
- Average mass366.429 Da
Roluperidone
CAS 359625-79-9
1937215-88-7 hcl
CYR-101
UNII-4P31I0M3BF
MIN-101
SYN
Roluperidone (former developmental code names MIN-101, CYR-101, MT-210) is a 5-HT2A and σ2 receptor antagonist that is under development by Minerva Neurosciences for the treatment of schizophrenia.[1][2][3][4] One of its metabolites also has some affinity for the H1 receptor.[2] As of May 2018, the drug is in phase III clinical trials.[5]
Minerva Neurosciences (following the merger of Cyrenaic and Sonkei Pharmaceuticals ), under license from Mitsubishi Tanabe Pharma , is developing roluperidone (MIN-101, CYR-101, MT-210), a dual 5-HT2A /sigma 2 antagonist, as a modified-release formulation, for the potential oral treatment of schizophrenia. In December 2017, a phase III trial was initiated in patients with negative symptoms of schizophrenia. By March 2020, Minerva had filed an IND for apathy in dementia.
Schizophrenia is a complex, challenging, and heterogeneous psychiatric condition, affecting up to 0.7% of the world population according to the World Health Organization (WHO, 2006). Patients suffering with schizophrenia present with a range of symptoms, including: positive symptoms, such as delusions, hallucinations, thought disorders, and agitation; negative symptoms, such as mood flatness and lack of pleasure in daily life; cognitive symptoms, such as the decreased ability to understand information and make decisions, difficulty focusing, and decreased working memory function; and sleep disorders.
The etiology of schizophrenia is not fully understood. A major explanatory hypothesis for the pathophysiology of schizophrenia is the Dopamine (DA) hypothesis, which proposes that hyperactivity of DA transmission is responsible for expressed symptoms of the disorder. This hypothesis is based on the observation that drugs effective in treating schizophrenia share the common feature of blocking DA D2 receptors. However, these so-called typical antipsychotics are associated with a very high incidence of extrapyramidal symptoms (EPS). Furthermore, negative symptoms and cognitive impairment are considered relatively unresponsive to typical antipsychotics.
Most currently approved therapies for schizophrenia show efficacy primarily in the management of positive symptoms. An estimated 4.2 million people suffered from schizophrenia in 2012 in the United States and the five major European Union markets. Of those, an estimated 48% experienced predominantly negative symptoms and 80% suffered from cognitive impairment. In addition, about 50% of patients with schizophrenia experience sleep disorders, which can further exacerbate both positive and negative symptoms.
The introduction of the so-called atypical antipsychotics in the last decade represented a significant advance in the treatment of schizophrenia. Although these atypical antipsychotics differ widely in chemical structure and receptor-binding profiles, they share a characteristic of potent antagonism of the Serotonin (5-hydroxytryptamine) type 2 receptor (5-HT2A). A high 5-HT2A:D2 affinity ratio is thought to substantially reduce the liability for inducing EPS, compared with typical antipsychotics.
However, many patients are still treatment-noncompliant despite the advantage of atypical antipsychotics of tolerability. Although the risk of EPS is clearly lower with the atypical antipsychotics, the high doses required with some atypical antipsychotics are likely to result in an increased incidence of EPS and require concomitant medications such as antiparkinson drugs.
In addition to EPS, antipsychotic medications cause a broad spectrum of side effects including sedation, anticholinergic effects, prolactin elevation, orthostatic hypotension, weight gain, altered glucose metabolism, and QTc prolongation. These side effects can affect patients’ compliance with their treatment regimen. It should be noted that noncompliance with treatment regimen is a primary reason for relapse of the disease.
Although atypical antipsychotics offer advantages over typical antipsychotics in terms of symptom alleviation and side effect profile, these differences are generally modest. A certain population of patients still remains refractory to all currently available antipsychotics. Newer agents to address these issues continue to be sought.
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Roluperidone hydrochloride | WFL7TF8DTP | 1937215-88-7 | NZKANSJXJCILHS-UHFFFAOYSA-N |
PATENT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2001064670
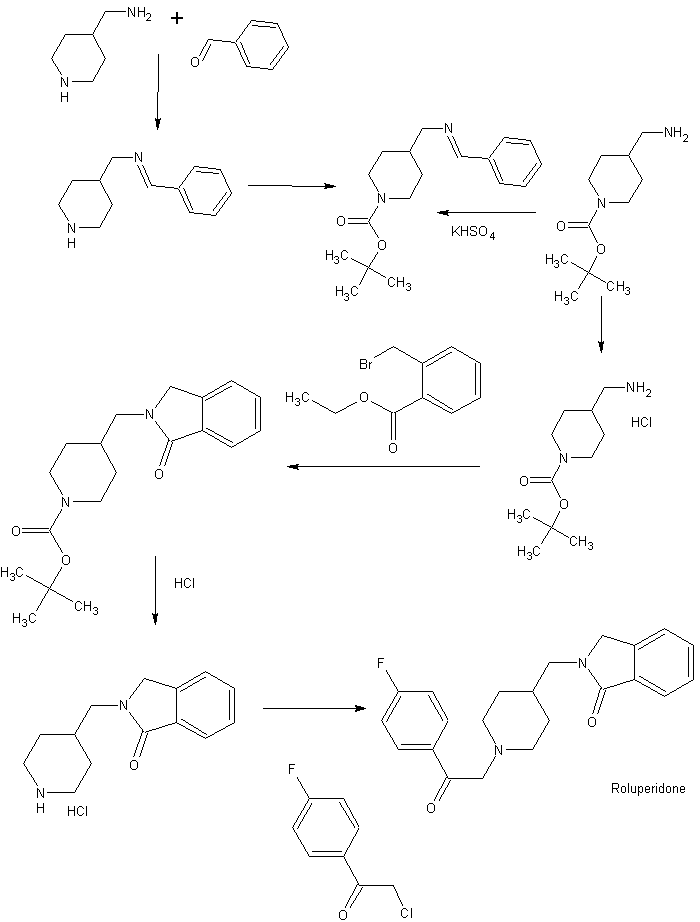
Example 1: 2-[[1- [2–fluorophenyl) -2-oxotyl] piperidine –4-yl] methyl] isoindrin-hydrochloride (Compound 1 in Table 1)
a) tert-Butyl 4-aminomethylpiperidine-carpoxylate hydrochloride’salt
4-Aminomethylpiperidin 5. 71g as a starting material
Tert-Butyl 4-aminomethylbiperidine-power reportage was synthesized according to the method described in Synthetic Commun., 22 (16), 2357-2360 (1992). This compound was dissolved in 80 ml of ethyl acetate, 4N ethyl monoacetate hydrochloride was added, and the mixture was stirred. Precipitated solid
Was collected to obtain 10.27 g (yield 82%) of the indicated compound. At melting point 236-240.
Ή-NMR (DMS0-d 6 ): 8.00 (3H, s), 3. 92 (2H, br d, J = 12.6), 2.68 H, m), 1.77- 1. 65 (3H, m), 1.39 (9H, s), 1.02 (2H, m) b) 2-Bromomethylbenzoic acid etyl ester
2-Methylbenzoic acid etyl ester (2.00 g, 11.9 mmol) is dissolved in carbon tetrachloride (60 ml), and N-promosucciimide (2.56 g, 14.4 mmo 1) and a catalytic amount of benzoyl peroxide are added to the solution. In addition, heat reflux. After 1 hour, the reaction mixture was cooled to room temperature, hexan (40 m was added, the insoluble material was filtered off, and the filtrate was distilled off under reduced pressure to obtain 3.16 g of the indicated compound as a yellow oil. It was used for the next reaction without purification as it was.
c) tert-Butyl 4- (1-oxoisoindrin-2 -ylmethyl) piperidine-1 -carpoxylate
Add 3.15 g of the compound obtained in Example lb and the compound (3.00 g, 12. Ommol) obtained in Example la to dimethylformamide (30πΠ), and stir at room temperature with trietylamine (3.5 ml, 25 mmol). ) Is added and stirred at the same temperature for 17 hours. Water is added to the reaction mixture, and the mixture is extracted with a mixed solvent of etyl hexane vinegar. The organic layer is washed with 10% aqueous quenic acid solution, water, sodium bicarbonate solution, and saturated brine, and dried with magnesium sulfate. The insoluble material was filtered, the filtrate was distilled off under reduced pressure, and the obtained oil was purified by silicon gel column chromatography (etyl-hexan acetate). I got it as a thing.
Ή-NMR (CDC1 3 ): 7.85 (1H, d, J = 7.5), 7.4-7.6 (3Η, m),
4.41 (2H, s), 4.0-4.2 (2H, m), 3.4-3.6 (2H, m), 2.6-2.8 (2H, m), 1.8-2.0 (1H, m), 1.5 -1.7 (4H, m), to 45 (9H, s)
d) 2- (Piperidine -4 -Ilmethyl) Isondrin -1 -On Hydrochloride
The compound (1.6 lg, 4.87 mmol) obtained in Example 1c is dissolved in methylene chloride (5 ml) and ethanol (lm mixed solvent, and at room temperature, 4 standard ethyl acetate solvent (5 ml, 20 mmol) is added. Stir at warm temperature for 1 hour and filter the precipitated solid. The obtained solid was washed with ethanol acetate and then dried under reduced pressure to give the indicated compound 7260 ^ (yield 56%) as a colorless solid. ..
Ή-NMR (DMS0-d 6 ): 8. 83 (1H, brs), 8. 53 (1H, brs), 7. 4-7. 7 (4 Η, m), 4. 50 (2H, s), 3. 44 (2H, d, J = 7.2), 3. 2-3. 3 (2H, i), 2. 7-2.9 (2H, m), 1. 9-2.1 (1H) , m), 1. 6-1. 8 (2H, m), 1. 3-1. 5 (2H, m)
e) 2- [Π_ [2- (4-Fluo-mouth phenyl) -2-oxotil] Piperidin –4-yl] Methyl] Isoindrin-卜 on
Add the compounds obtained in Example Id (518 mg, 1. 94 mmo and 2-cloucet -4, -fluoroacetophenone (358 mg, 2.07 mmol) to dimethylform amamide (12 ml) with stirring at room temperature. Add trietylamine (575 1, 4. 13 mmol). After stirring at the same temperature for 4 hours, add water to the reaction solution and extract with ethyl acetate. The organic layer is washed with water and saturated saline and sodium sulfate. Dry with thorium. Filter the insoluble material and concentrate the filtrate under reduced pressure to obtain 0.70 g of orange oil. Add hexane to the obtained oil to solidify. Filter this. By drying under reduced pressure, 551 mg (yield 77%) of the notation compound was obtained as a pale yellow solid.
! H-NMR (CDC1 3 ): 8.0-8 . 1 (2H, m), 7. 85 (1H, d = 7.2), 7.4-7. 55 (3 Η, m), 7.1 2 ( 2H, t), 4. 41 (2H, s), 3. 73 (2H, s), 3.51 (2H, d, J = 7.5), 2. 9-3. 0 (2H, m) , 2. 1-2. 2 (2H, m), 1. 4-19.9 (5H, m)
f) 2- [Π- [2- (4 -Fluolophenyl) -2 -Oxoetyl] Piperidin –4-yl] Methyl] Isoindoline-Piol hydrochloride
The compound (550 mg, 1.5 Ommo 1) obtained in Example le was used as an etano.
Dissolve in (2 ml) and add 4 specified ethyl hydrochloride solvent (2 ml, 8 imol) at room temperature and stir at the same temperature for 15 minutes. Ethyl acetate (10 ml) is added to the reaction solution, and the precipitated solid is filtered. The obtained solid is washed with ethyl acetate and then dried under reduced pressure to obtain 364 mg of white powder. This was recrystallized from ethanol monoacetate to give 246 mg (yield 41%) of the notation compound as a colorless solid. At melting point 182-188.
Ή-NMR (DMS0-d 6 ): 9.93 (1H, brs), 8.0-8. 2 (2H, m), 7.4-7.7 (6 Η, m), 4. 9-5.1 (2H, m), 4.53 (2H, s), 2.9-3.6 (6H, m), 1.6-2.2 (5H, m)
PATENT
https://patents.google.com/patent/US7166617B2/en
Example 12-[[1-[2-(4-Fluorophenyl)-2-oxoethyl]piperidin-4-yl]methyl]isoindolin-1-one hydrochloride (Compound 1 in Table 1)a) tert-Butyl 4-aminomethylpiperidine-1-carboxylate hydrochloride
By using 4-aminomethylpiperidine 5.71 g as a starting material, tert-butyl 4-aminomethylpiperidine-1-carboxylate was prepared according to the method described in Synthetic Commun., 22(16), 2357–2360 (1992). The resulting compound was dissolved in 80 ml of ethyl acetate, and the solution was added with 4N hydrogen chloride-ethyl acetate and stirred. The precipitated solids were collected by filtration to obtain the title compound (10.27 g, yield: 82%).
Melting point: 236–240° C. 1H-NMR(DMSO-d6): 8.00(3H,s), 3.92(2H, br d, J=12.6), 2.68(4H, m), 1.77–1.65(3H, m), 1.39(9H, s), 1.02(2H, m)
b) 2-Bromomethylbenzoic acid ethyl ester
2-Methylbenzoic acid ethyl ester (2.00 g, 11.9 mmol) was dissolved in carbon tetrachloride (60 ml), and the solution was added with N-bromosuccinimide (2.56 g, 14.4 mmol) and a catalytic amount of benzoylperoxide and then heated under reflux. After one hour, the reaction mixture was cooled to room temperature and added with hexane (40 ml) to remove insoluble solids by filtration. The filtrate was evaporated under reduced pressure to obtain the title compound 3.16 g as yellow oil. the product was used in the next reaction without purification.
c) tert-Butyl 4-(1-oxoisoindolin-2-yl-methyl)piperidine-1-carboxylate
The compound obtained in Example 1b (3.15 g), and the compound obtained in Example 1a (3.00 g, 12.0 mmol) were added in dimethylformamide (30 ml). The mixture was added with triethylamine (3.5 ml, 25 mmol) with stirring at room temperature, and then stirring was continued for 17 hours at the same temperature. Water was added to the reaction mixture and extracted with a mixed solvent of ethyl acetate-hexane. The organic layer was washed with 10% aqueous citric acid solution, water, aqueous sodium bicarbonate solution, and then with saturated brine and the dried over magnesium sulfate. Insoluble solids were removed by filtration, and the filtrate was evaporated under reduced pressure. The resulting oil was purified by silica gel column chromatography (ethyl acetate-hexane) to obtain the title compound as yellow oil (yield: 41%)
1H-NMR(CDCl3): 7.85(1H,d,J=7.5), 7.4–7.6(3H,m), 4.41(2H,s), 4.0–4.2(2H,m), 3.4–3.6(2H,m), 2.6–2.8(2H,m), 1.8–2.0(1H,m), 1.5–1.7(4H,m), 1.45(9H,s)
d) 2-(Piperidin-4-yl-methyl)isoindolin-1-one hydrochloride
The compound obtained in Example 1c (1.61 g, 4.87 mmol) was dissolved in a mixed solvent of methylene chloride (5 ml) and ethanol (1 ml) and the solution was added with 4N hydrochloric acid in ethyl acetate (5 ml, 20 mmol) at room temperature. The mixture was stirred at the same temperature for 1 hour, and the precipitated solids were collected by filtration. The resulting solids were washed with ethyl acetate and then dried under reduced pressure to obtain the title compound as colorless solid (726 mg, yield: 56%).
1H-NMR(DMSO-d6): 8.83(1H,brs), 8.53(1H,brs), 7.4–7.7(4H,m), 4.50(2H,s), 3.44(2H,d,J=7.2), 3.2–3.3(2H,m), 2.7–2.9(2H,m), 1.9–2.1(1H,m), 1.6–1.8(2H,m), 1.3–1.5(2H,m)
e) 2-[[1-[2-(4-Fluorophenyl)-2-oxoethyl]piperidin-4-yl]methyl]isoindolin-1-one
The compound obtained in Example 1d (518 mg, 1.94 mmol) and 2-chloro-4′-fluoroacetophenone (358 mg, 2.07 mmol) was added to dimethylformamide (12 ml), and the solution was added with triethylamine (575 μl, 4.13 mmol) with stirring at room temperature. Stirring was continued at the same temperature for 4 hours, and then the reaction mixture was added with water and extracted with ethyl acetate. The organic layer was washed with water and then with saturated brine, and then dried over sodium sulfate. Insoluble solids were removed by filtration and the filtrate was evaporated under reduced pressure to obtain orange oil (0.70 g). The resulting oil was solidified by adding hexane, and the solids were collected by filtration and dried under reduced pressure to obtain the title compound as pale yellow solid (551 mg, yield: 77%).
1H-NMR(CDCl3): 8.0–8.1(2H,m), 7.85(1H,d=7.2), 7.4–7.55(3H,m), 7.12(2H,t), 4.41(2H,s), 3.73(2H,s), 3.51(2H,d,J=7.5), 2.9–3.0(2H,m), 2.1–2.2(2H,m), 1.4–1.9(5H,m)
f) 2-[[1-[2-(4-Fluorophenyl)-2-oxoethyl]piperidin-4-yl]methyl]isoindolin-1-one hydrochloride
The compound obtained in Example 1e (550 mg, 1.50 mmol) was dissolved in ethanol (2 ml), and the solution was added with 4N hydrochloric acid in ethyl acetate (2 ml, 8 mmol) at room temperature, and stirring was continued at the same temperature for 15 minutes. The reaction mixture was added with ethyl acetate (10 ml) and the precipitated solids were collected by filtration. The resulting solids were washed with ethyl acetate and then dried under reduced pressure to obtain white powder (364 mg). The product was recrystallized from ethanol-ethyl acetate to obtain the title compound as colorless solid (246 mg, yield: 41%)
Melting point: 182–188° C. 1H-NMR(DMSO-d6): 9.93(1H,brs), 8.0–8.2(2H,m), 7.4–7.7(6H,m), 4.9–5.1(2H,m), 4.53(2H,s), 2.9–3.6(6H,m), 1.6–2.2(5H, m)
PATENT
https://patents.google.com/patent/US9458130B2/en?oq=9%2c458%2c130+US
PATENT
WO-2020264486
Novel crystalline form of roluperidone HCL (designated as form 4) as 5-HT 2a receptor antagonist useful for treating schizophrenia.
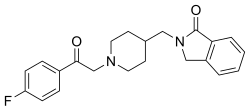
Roluperidone has the chemical name 2-({ l-[2-(4-Fluorophenyl)-2-oxoethyl]-4-piperidinyl}methyl)-l-isoindolinone. Roluperidone has the following chemical structure:
[0003] Roluperidone is reported to be a drug candidate with equipotent affinities for 5-hydroxytryptamine-2A (5-HT2A) and sigma2 and, at lower affinity levels, al -adrenergic receptors. A pivotal Phase 3 clinical trial is ongoing with roluperidone as a monotherapy for negative symptoms in patients diagnosed with schizophrenia.
[0004] Roluperidone is known from U.S. Patent No. 7,166,617.
[0005] Solid state form of 2-((l-(2-(4-Fluorophenyl)-2-oxoethyl)piperidin-4-yl)methyl)isoindolin-l-o-ne monohydrochloride dihydrate is known from U.S. Patent No.9,458,130.
Examples
[00113] Roluperidone can be prepared according to the procedure described in U.S. Patent No. 7,166,617.
Example 1: Preparation of Roluperidone HC1
[00114] 2.02 grams of Roluperidone was dissolved in acetone (80 mL). 2.76 mL of HC1 (2M) was added to the solution. The obtained suspension was stirred for 21 hours at 10°C and then filtered over black ribbon filter paper under vacuum. Obtained solid was analyzed by PXRD.
References
- ^ Mestre TA, Zurowski M, Fox SH (April 2013). “5-Hydroxytryptamine 2A receptor antagonists as potential treatment for psychiatric disorders”. Expert Opinion on Investigational Drugs. 22 (4): 411–21. doi:10.1517/13543784.2013.769957. PMID 23409724.
- ^ Jump up to:a b Ebdrup BH, Rasmussen H, Arnt J, Glenthøj B (September 2011). “Serotonin 2A receptor antagonists for treatment of schizophrenia”. Expert Opinion on Investigational Drugs. 20 (9): 1211–23. doi:10.1517/13543784.2011.601738. PMID 21740279.
- ^ Köster LS, Carbon M, Correll CU (December 2014). “Emerging drugs for schizophrenia: an update”. Expert Opinion on Emerging Drugs. 19 (4): 511–31. doi:10.1517/14728214.2014.958148. PMID 25234340.
- ^ “Drug Development in Schizophrenia: Summary and Table”. Pharmaceutical Medicine. 28 (5): 265–271. 2014. doi:10.1007/s40290-014-0070-6. ISSN 1178-2595.
- ^ “Roluperidone – Minerva Neurosciences”. Adis Insight. Springer Nature Switzerland AG.
 |
|
| Clinical data | |
|---|---|
| Other names | MIN-101; CYR-101; MT-210 |
| Routes of administration |
By mouth |
| Identifiers | |
| CAS Number | |
| PubChemCID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H23F2N2O2 |
| Molar mass | 385.435 g·mol−1 |
| 3D model (JSmol) | |
/////////////////Roluperidone, PHASE 3, ролуперидон , رولوبيريدون , 罗鲁哌酮 , CYR 101, UNII-4P31I0M3BF , MIN 101,
C1CN(CCC1CN2CC3=CC=CC=C3C2=O)CC(=O)C4=CC=C(C=C4)F














