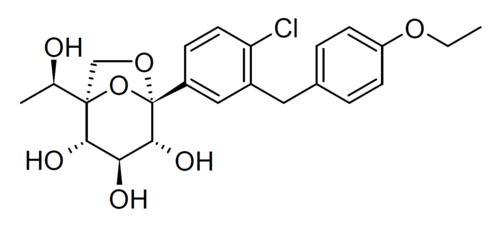
Rongliflozin
Olorigliflozin, 6FP3NST6ZQ, DJT1116PG
Cas 2035989-50-3
450.9 g/mol, C23H27ClO7
(1R,2S,3S,4R,5S)-5-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-1-[(1R)-1-hydroxyethyl]-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol
- (1R,2S,3S,4R,5S)-5-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-1-((R)-1-hydroxyethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol
- 1,6-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-5-C-[(1R)-1-hydroxyethyl]-beta-L-idopyranose
- beta-L-Idopyranose, 1,6-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-5-C-[(1R)-1-hydroxyethyl]-
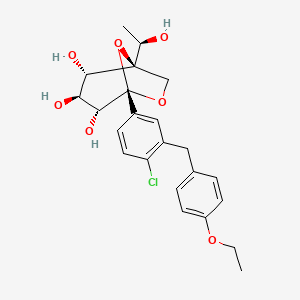
Rongliflozin 화학구조
CAS No. : 2648020-91-9
| MW | 602.55 |
|---|---|
| MF | C23H27ClO7.C5H7NO3.5/4H2O |
- OriginatorHEC Pharm
- DeveloperSunshine Lake Pharma
- ClassAntihyperglycaemics; Small molecules
- Mechanism of ActionSodium-glucose transporter 2 inhibitors
- PreregistrationType 2 diabetes mellitus
- 04 Sep 2025Chemical structure information added.
- 31 Dec 2023Preregistration for Type 2 diabetes mellitus in China (PO), in December 2023
- 31 Dec 2023Efficacy and adverse events data from a phase IIIa trial in Type 2 diabetes mellitus released by Sunshine Lake Pharma, before December 2023
Rongliflozin is an SGLT2 inhibitor developed as a potential treatment for diabetes.[1][2]
Rongliflozin (DJT1116PG) is a selective and orally active inhibitor of sodium-glucose co-transporter-2 (SGLT-2). Rongliflozin can be used for the research of type 2 diabetes mellitus (T2DM).
PAT
- (1R,2S,3S,4R,5S)-5-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-1-((R)-1-hydroxyethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol
- 1,6-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-5-C-[(1R)-1-hydroxyethyl]-beta-L-idopyranose
- beta-L-Idopyranose, 1,6-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-5-C-[(1R)-1-hydroxyethyl]-
- Complexes of glucopyranosyl derivatives and methods for their preparation and usePublication Number: JP-2018535237-APriority Date: 2015-11-27
- Complex of a glucopyranosyl derivative and preparation method and use thereofPublication Number: US-10555930-B2Priority Date: 2015-11-27Grant Date: 2020-02-11
- Complex of a glucopyranosyl derivative and preparation method and use thereofPublication Number: US-2018344689-A1Priority Date: 2015-11-27
- A complex of a glucopyranosyl derivative and preparation method and use thereofPublication Number: WO-2017088839-A1Priority Date: 2015-11-27
- Glucopyranosyl derivative complex and its preparation method and usePublication Number: JP-6916180-B2Priority Date: 2015-11-27Grant Date: 2021-08-11
- Preparation method and intermediate of glucopyranosyl derivativesPublication Number: CN-113195510-BPriority Date: 2019-01-08Grant Date: 2022-12-23
- Crystalline forms of glucopyranosyl derivativesPublication Number: CN-107778336-BPriority Date: 2016-08-24Grant Date: 2022-09-27
- Glucopyranosyl derivative compound, preparation method and applicationPublication Number: CN-106810582-APriority Date: 2015-11-27
- Glucopyranosyl derivative compound, preparation method and applicationPublication Number: CN-106810582-BPriority Date: 2015-11-27Grant Date: 2019-12-31
- A complex of a glucopyranosyl derivative and preparation method and use thereofPublication Number: EP-3371199-A1Priority Date: 2015-11-27
- Method for preparing glucopyranosyl derivatives and intermediates thereofPublication Number: WO-2022007838-A1Priority Date: 2020-07-08
- Method for preparing glucopyranosyl derivatives and intermediates thereofPublication Number: EP-4178970-A1Priority Date: 2020-07-08
- Method for preparing glucopyranosyl derivatives and intermediates thereofPublication Number: US-2023250121-A1Priority Date: 2020-07-08
- Preparation methods of glucopyranosyl derivatives and intermediates thereofPublication Number: CN-113912567-BPriority Date: 2020-07-08Grant Date: 2024-01-16
- Preparation method for glucopyranosyl derivative and intermediate thereofPublication Number: WO-2020143653-A1Priority Date: 2019-01-08
- Composition and use of sglt-2 inhibitor and angiotensin receptor blockersPublication Number: WO-2022036506-A1Priority Date: 2020-08-17
- Composition and use of sglt-2 inhibitor and angiotensin receptor blockersPublication Number: EP-4197543-A1Priority Date: 2020-08-17
- Compositions of SGLT-2 inhibitors and angiotensin receptor antagonists and uses thereofPublication Number: KR-20230057388-APriority Date: 2020-08-17
- Composition and application of SGLT-2 inhibitor and angiotensin receptor blockerPublication Number: CN-116490178-APriority Date: 2020-08-17
- Composition and use of sglt-2 inhibitor and angiotensin receptor blockersPublication Number: US-2023346817-A1Priority Date: 2020-08-17
- Nintedanib targeted combinationPublication Number: CN-118021812-APriority Date: 2023-12-30
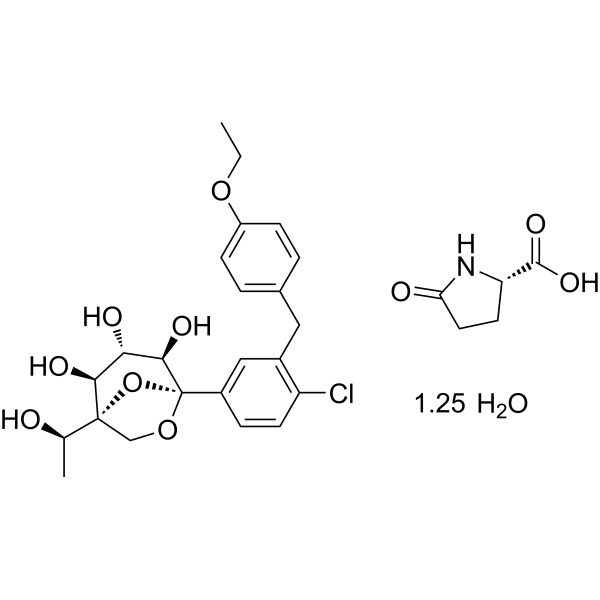
- Preparation method of L-pyroglutamic acid co-crystal of glucopyranosyl derivativesPublication Number: CN-115141235-APriority Date: 2021-03-30
- Preparation method of L-pyroglutamic acid cocrystal of pyranose glucopyranose derivativePublication Number: CN-115141235-BPriority Date: 2021-03-30Grant Date: 2024-08-09
- Fixed-dose combination of sglt-2 inhibitor and angiotensin converting enzyme inhibitor, and use thereofPublication Number: WO-2022104621-A1Priority Date: 2020-11-19
- Compositions and uses of fixed-dose SGLT-2 inhibitors and angiotensin-converting enzyme inhibitorsPublication Number: CN-116234545-APriority Date: 2020-11-19
SYN
https://pubs.rsc.org/en/content/articlelanding/2021/ce/d1ce01305j/unauth
Rongliflozin L-pyroglutamic acid, a highly active SGLT-2 inhibitor cocrystal discovered and developed by our group, is currently undergoing clinical trials for the treatment of diabetes. Here, we report and design a simple and robust process to obtain a single and pure crystalline form I (1) of the cocrystal, containing Rongliflozin (2) with L-pyroglutamic acid (L-PA), based on coformer-induced purification (CoIP). Extensive experiments showed that the addition of L-pyroglutamic acid in the eluent was key to suppression of the dissociation equilibrium of the cocrystal during lessivation, with high efficiency. Importantly, based in this profile, this process exhibited strong robustness and margin of safety at multigram and multikilogram scales
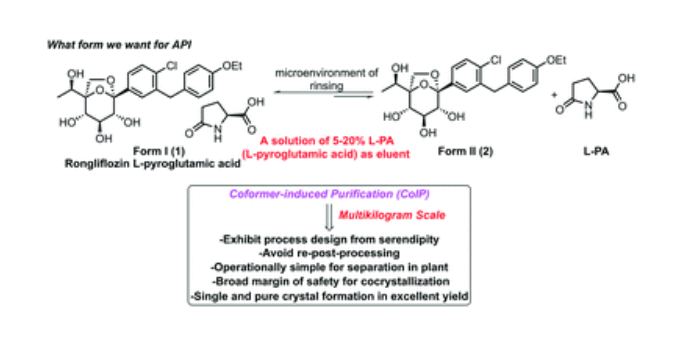
Kilogram scale Process of 1
A mixture of (1R,2S,3S,4R,5S)-5-(4-chloro-3-(4-ethoxybenzyl) phenyl)-1-((R)-1-
hydroxyethyl)-6,8-dioxabicyclo [3.2.1] octane-2,3,4-triol ethanolate form III (3) (23.45 kg, 47.3
mol), L-pyroglutamic acid (24.31 kg, 4.0 equiv.), EtOH (35.9 L) and H2O (70 L) was added into a
300 L reactor at room temperature. The slurry was heated to 65 °C and stirred until it is clear. The
clear solution was cooled to 35±5 °C typically. Seed crystal form I (1) (0.70 kg, 3% g/g) was added
when the solution was cooled to 34 °C and maintained for 1.5 h. Gradually, the slurry was cool to
30 °C and 25 °C in 3 hours, and finally stirred at 25 °C for 24 h. The slurry was collected on a
centrifuge filter. The filter cake was washed with a mixed solution of EtOH (31.3 L)/H2O (62.7 L)
with L-pyroglutamic acid (1.64 kg, 7% g/g) pre-cooled to -15°C. The wet cake was dried under
vacuum at 45 °C for 8 h. Pure cocrystal form I (1) was obtained as a white solid (24.91 kg, yield
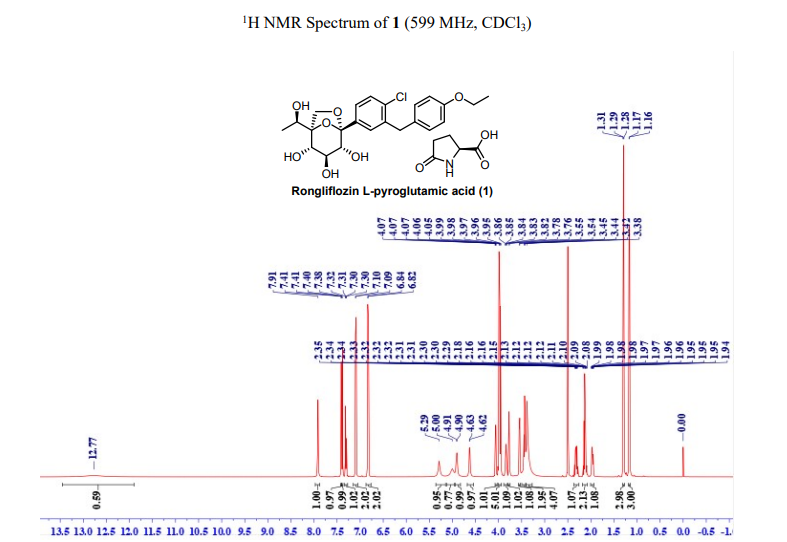
91%). MP (DSC onset) = 96.91 ℃. 1H NMR (599 MHz, DMSO-d6) δ 12.77 (br, 1H), 7.91 (s, 1H),
7.41 (d, J = 2.0 Hz, 1H), 7.39 (d, J = 12.0 Hz, 1H), 7.31 (dd, J = 12.0, 2.0 Hz, 1H), 7.10 (d, J = 2.0
Hz , 2H), 6.83 (d, J = 2.0 Hz, 2H), 5.29 (s, 1H), 5.00 (s, 1H), 4.91 (d, J = 6.7 Hz, 1H), 4.63 (d, J =
6.1 Hz, 1H), 4.06 (dd, J = 12.0, 6.0 Hz, 1H), 3.99– 3.95 (m, 5H), 3.84 (p, J = 6.0 Hz, 1H), 3.77 (d,
J = 12.0 Hz, 1H), 3.55 (d, J = 6.0 Hz, 1H), 3.44 (t, J = 12.0 Hz, 2H), 3.38 (s, 4H), 2.35-2.29 (m,
1H), 2.18-2.08 (m, 2), 1.99-1.94 (m, 1H), 1.29 (t, J = 12.0 Hz, 3H), 1.17 (d, J = 6.0 Hz, 3H). 13C
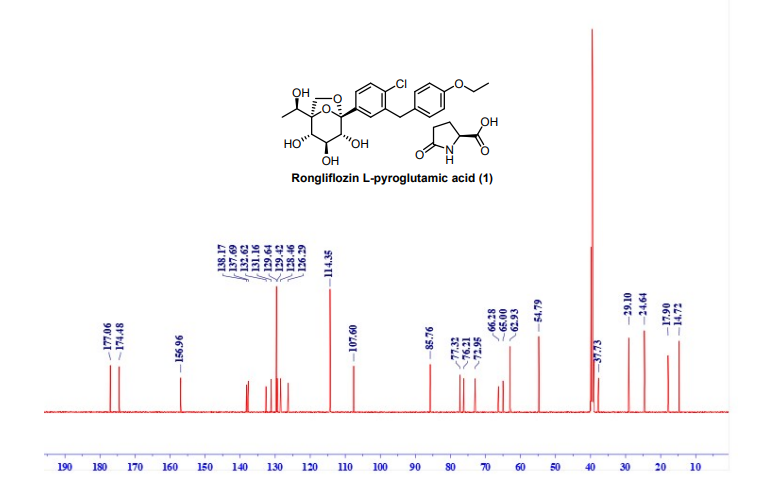
NMR (151 MHz, DMSO-d6) δ 177.06, 174.48, 156.96, 138.17, 137.69, 131.16, 129.64, 129.42,
128.46, 126.29, 114.35, 107.60, 85.76, 77.32, 76.21, 72.95, 66.28, 65.00, 62.93, 54.79, 37.73, 29.10,
24.64, 17.90, 14.72. HRMS: (ESI) Calcd for C23H27ClO7 [M+NH4]+: 468.1784, C5H7NO3 [M+H]+
:130.0499; Found: 468.1774, 130.0490 respectively. IR (KBr, cm-1): 3257, 2986, 2927, 1750, 1648,
1513, 1476, 1371, 1264, 1239, 1223, 1206, 1088, 1061, 821
13C NMR



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Zhang H, Liu J, Zhu X, Li X, Chen H, Wu M, et al. (May 2020). “A Phase I Study on the Pharmacokinetics and Pharmacodynamics of DJT1116PG, a Novel Selective Inhibitor of Sodium-glucose Cotransporter Type 2, in Healthy Individuals at Steady State”. Clinical Therapeutics. 42 (5): 892–905.e3. doi:10.1016/j.clinthera.2020.03.007. PMID 32265061.
- Zhang H, Zhu X, Li X, Chen H, Wu M, Li C, et al. (February 2020). “Pharmacokinetics and pharmacodynamics of rongliflozin, a novel selective inhibitor of sodium-glucose co-transporter-2, in people with type 2 diabetes mellitus”. Diabetes, Obesity & Metabolism. 22 (2): 191–202. doi:10.1111/dom.13887. PMID 31588657.
| Legal status | |
|---|---|
| Legal status | Investigational |
| Identifiers | |
| IUPAC name | |
| CAS Number | 2035989-50-3 |
| PubChem CID | 122660464 |
| UNII | 6FP3NST6ZQ |
| ChEMBL | ChEMBL5314927 |
| Chemical and physical data | |
| Formula | C23H27ClO7 |
| Molar mass | 450.91 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
/////////////Rongliflozin, diabetes, Olorigliflozin, 6FP3NST6ZQ, 2035989-50-3, DJT1116PG, DJT 1116PG,














