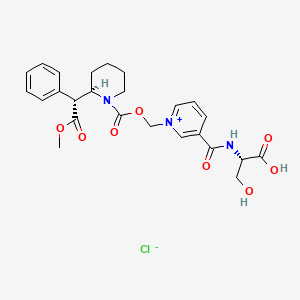
Serdexmethylphenidate
- Molecular FormulaC25H30ClN3O8
- Average mass535.974 Da
CAS
Azstarys, FDA APPROVED, 3/2/2021, Products on NDA 212994, Type 1 – New Molecular Entity and Type 4 – New Combination
Serdexmethylphenidate Chloride (SDX), SDX or KP145
https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212994s000lbl.pdf
| Molecular Formula | C25H30ClN3O8 |
|---|---|
| Synonyms |
UNII-FN54BT298Y KP415 Cl Serdexmethylphenidate chloride FN54BT298Y Serdexmethylphenidate chloride (USAN) |
| Molecular Weight | 536 g/mol |
CAS 1996626-30-2
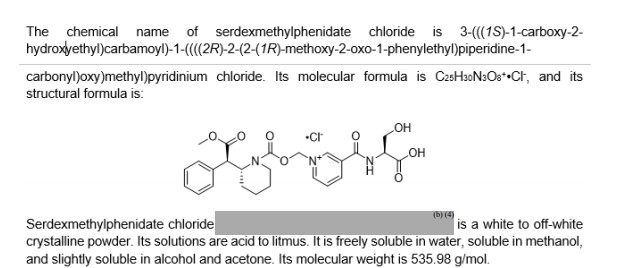
(2S)-3-hydroxy-2-[[1-[[(2R)-2-[(1R)-2-methoxy-2-oxo-1-phenylethyl]piperidine-1-carbonyl]oxymethyl]pyridin-1-ium-3-carbonyl]amino]propanoic acid;chloride
Serdexmethylphenidate is a derivative of dexmethylphenidate created by pharmaceutical company KemPharm. The compound is under investigation for the treatment of ADHD in children, adolescents, and adults as of 2020.[2] The drug was approved for medical use by the FDA in March, 2021. Serdexmethylphenidate is a prodrug which has a delayed onset of action and a prolonged duration of effects compared to dexmethylphenidate, its parent compound.
Formulations
Serdexmethylphenidate/dexmethylphenidate (Azstarys), a co-formulation of serdexmethylphenidate and dexmethylphenidate, was approved by the Food and Drug Administration (FDA) in March 2021, for the treatment of ADHD in those above six years of age. Co-formulation of serdexmethylphenidate with dexmethylphenidate allows for a more rapid onset of action while still retaining up to 13 hours of therapeutic efficacy.[3][4]
Due to serdexmethylphenidate’s delayed onset and prolonged duration of effects, several dosage forms containing serdexmethylphenidate have been investigated for use as long-acting psychostimulants in the treatment of ADHD. Under the developmental codename KP484, serdexmethylphenidate has been investigated as a “super-extended duration” psychostimulant, with therapeutic efficacy lasting up to 16 hours following oral administration. In 2011, MonoSol Rx entered into a partnership with KenPharm to develop oral films containing KP415.[5]
Abuse potential
The abuse potential of serdexmethylphenidate is theorized to be lower than other psychostimulants because serdexmethylphenidate is an inactive prodrug of dexmethylphenidate, and must undergo enzymatic metabolism prior to exerting any stimulant effects.[6] Common routes of administration used during the abuse of psychostimulants such as insufflation and intravenous injection have little impact on the pharmacokinetics and metabolism of serdexmethylphenidate and do not result in a faster onset of action.[7]
SYN
SYN
US 20200237742
Title
Abstract
(EN)
The present technology is directed to one or more compositions comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof. The present technology also relates to one or more compositions and oral formulations comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof. The present technology also relates to one or more methods of using compositions comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof. The present technology additionally relates to one or more pharmaceutical kits containing a composition comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof.
|
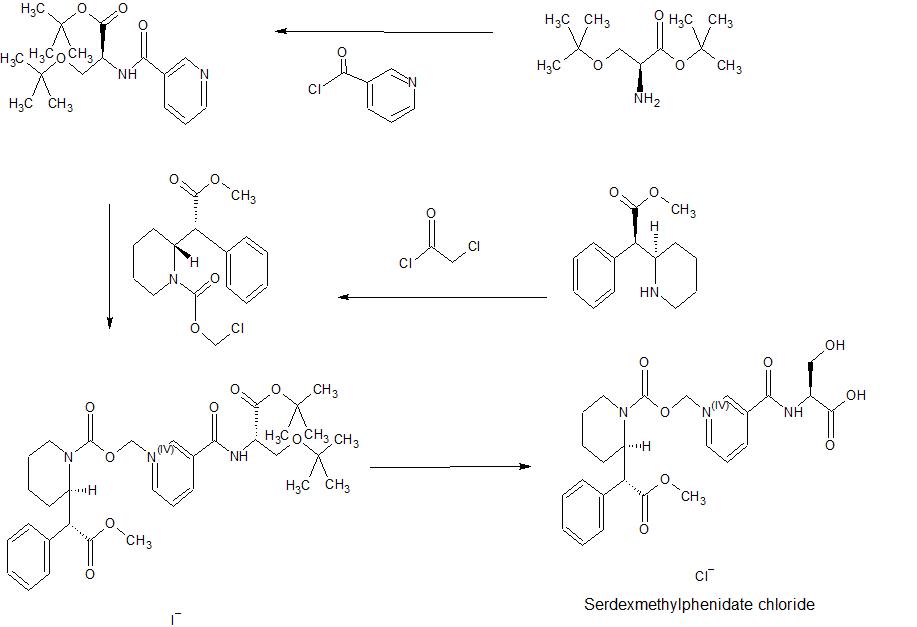
Synthetic Process for Making Serdexmethylphenidate
|
PATENT
US 20190381017
https://patentscope.wipo.int/search/en/detail.jsf?docId=US279620136&_cid=P10-KNB9P3-94207-1
Title
Abstract
(EN)
The present technology is directed to one or more compositions comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof. The present technology also relates to one or more compositions and oral formulations comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof. The present technology also relates to one or more methods of using compositions comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof. The present technology additionally relates to one or more pharmaceutical kits containing a composition comprising serdexmethylphenidate conjugates and unconjugated d-methylphenidate and/or a pharmaceutically acceptable salt thereof.
PATENT
WO 2019241019
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019241019&_cid=P10-KNB9QS-94329-1
PAT
WO 2018107131
WO 2018107132
References
- ^ “Azstarys Prescribing Information” (PDF). United States Food and Drug Administration. Retrieved 18 March 2021.
- ^ “KemPharm’s KP415 and Serdexmethylphenidate (SDX) Prodrug to be Featured in Multiple Sessions at the AACAP 2020 Virtual Meeting”. www.globenewswire.com.
- ^ Mickle T. “Prodrugs for ADHD Treatments: Opportunities & Potential to Fill Unmet Medical Needs” (PDF). Retrieved 15 November 2020.
- ^ Eric Bastings, MD (2 March 2021). “NDA 212994 Approval” (PDF). United States Food and Drug Administration. Retrieved 6 March 2021.
- ^ Van Arnum P (1 March 2012). “Meeting Solubility Challenges”. Pharmaceutical Technology. 2012 (2): S6–S8. Retrieved 15 November 2020.
- ^ Mickle T. “Prodrugs for ADHD Treatments: Opportunities & Potential to Fill Unmet Medical Needs” (PDF). Retrieved 15 November 2020.
- ^ Braeckman R (1 October 2018). “Human Abuse Potential of Intravenous Serdexmethylphenidate (SDX), A Novel Prodrug of D-Methylphenidate, in Recreational Stimulant Abusers”. Journal of the American Academy of Child & Adolescent Psychiatry. 57 (10): 176. doi:10.1016/j.jaac.2018.09.141. Retrieved 15 November 2020.
External links
- “Serdexmethylphenidate”. Drug Information Portal. U.S. National Library of Medicine.
 |
|
| Clinical data | |
|---|---|
| Other names | KP484 |
| License data | |
| Routes of administration |
By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 3%[1] |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H30ClN3O8 |
| Molar mass | 535.98 g·mol−1 |
| 3D model (JSmol) | |
| (verify) | |
//////////Serdexmethylphenidate, Azstarys, FDA 2021 APPROVALS 2021, SDX, KP 145,