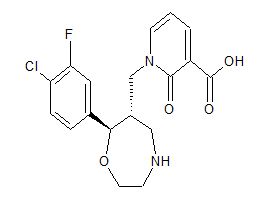
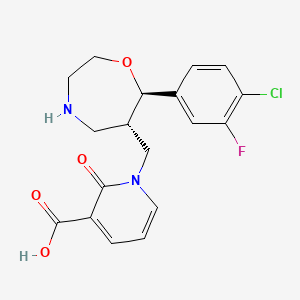
1-{[(6S,7R)-7-(4-Chloro-3-fluo
- Molecular Formula C18H18ClFN2O4
- Average mass 380.798 Da
CAS 1372185-97-1
CAS 1372180-09-0 hydrochloride
Peripherally selective noradrenaline reuptake inhibitor
 1-([(6S,7R)-7-(4-Chloro-3-fluorophenyl)-1,4-oxazepan-6-yl]methyl]-2-oxo-1,2-dihydropyridine-3-carboxylic acid monohydrochloride
1-([(6S,7R)-7-(4-Chloro-3-fluorophenyl)-1,4-oxazepan-6-yl]methyl]-2-oxo-1,2-dihydropyridine-3-carboxylic acid monohydrochloride
3-Pyridinecarboxylic acid, 1-[[(6S,7R)-7-(4-chloro-3-fluorophenyl)hexahydro-1,4-oxazepin-6-yl]methyl]-1,2-dihydro-2-oxo-, hydrochloride (1:1)
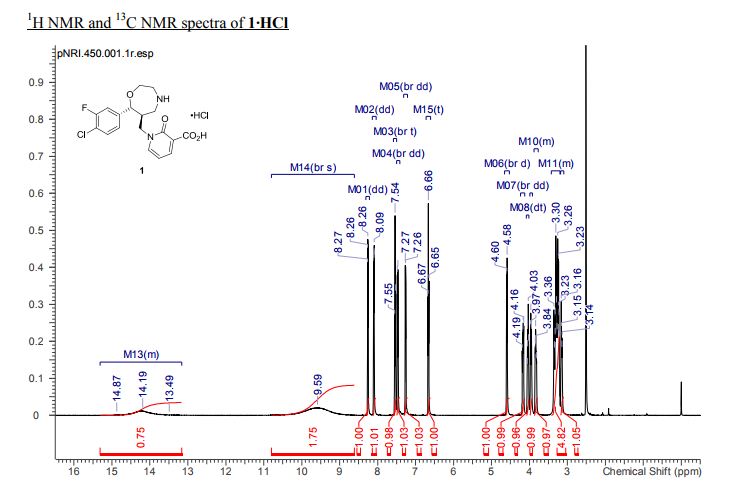
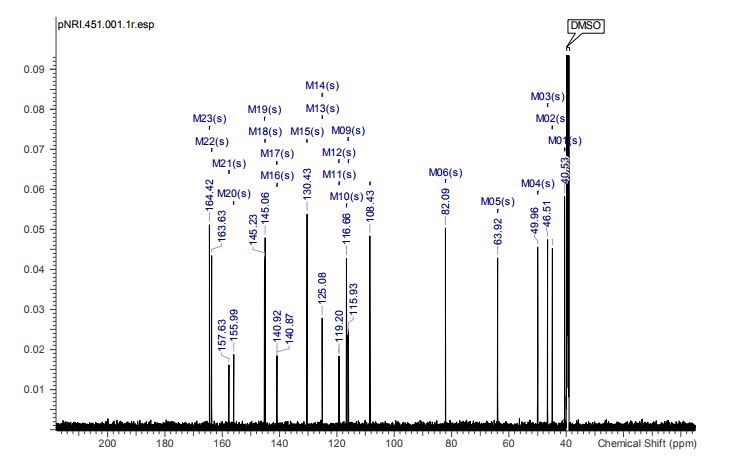
1-{[(6S,7R)-7-(4-Chloro-3-fluorophenyl)-1,4-oxazepan-6-yl]methyl}-2-oxo-1,2-dihydropyridine-3-carboxylic Acid Hydrochloride (1:1) (1·HCl)
TAKEDA PHARMACEUTICAL COMPANY LIMITED [JP/JP]; 1-1, Doshomachi 4-chome, Chuo-ku, Osaka-shi, Osaka 5410045 (JP)
ISHICHI, Yuji; (JP).
YAMADA, Masami; (US).
KAMEI, Taku; (JP).
FUJIMORI, Ikuo; (US).
NAKADA, Yoshihisa; (JP).
YUKAWA, Tomoya; (JP).
SAKAUCHI, Nobuki; (JP).
OHBA, Yusuke; (JP).
TSUKAMOTO, Tetsuya; (JP)
Paper
Development of a Practical Synthesis of a Peripherally Selective Noradrenaline Reuptake Inhibitor Possessing a Chiral 6,7-trans-Disubstituted-1,4-oxazepane as a Scaffold
Abstract
A practical synthesis of a peripherally selective noradrenaline reuptake inhibitor that has a chiral 6,7-trans-disubstituted-1,4-oxazepane as a new class of scaffold is described. The amino alcohol possessing the desired stereochemistry was obtained with excellent dr and ee, starting from a commercially available aldehyde via a Morita–Baylis–Hillman reaction, Michael addition, isolation as maleic acid salt, reduction, and diastereomeric salt formation with (+)-10-camphorsulfonic acid. The desired single stereoisomer obtained at an early stage of the synthesis was used for seven-membered ring formation in fully telescoped processes, providing the chiral 6,7-trans-disubstituted-1,4-oxazepane efficiently. In addition to controls of dr and ee of the chiral 1,4-oxazepane, and control of N,O-selectivity in SN2 reaction of the intermediate mesylate with a pyridone derivative, finding appropriate intermediates that were amenable to isolation and upgrade of purity enabled a practical chiral HPLC separation-free, column chromatograph-free synthesis of the drug candidate with excellent chemical and optical purities in a higher overall yield.
PATENT
https://www.google.com/patents/WO2012046882A1?cl=zh
PAPER
Volume 24, Issue 16, 15 August 2016, Pages 3716–3726
http://www.sciencedirect.com/science/article/pii/S0968089616304382
Abstract
Peripheral-selective inhibition of noradrenaline reuptake is a novel mechanism for the treatment of stress urinary incontinence to overcome adverse effects associated with central action. Here, we describe our medicinal chemistry approach to discover a novel series of highly potent, peripheral-selective, and orally available noradrenaline reuptake inhibitors with a low multidrug resistance protein 1 (MDR1) efflux ratio by cyclization of an amide moiety and introduction of an acidic group. We observed that the MDR1 efflux ratio was correlated with the pKa value of the acidic moiety. The resulting compound 9exhibited favorable PK profiles, probably because of the effect of intramolecular hydrogen bond, which was supported by a its single-crystal structure. The compound 9, 1-{[(6S,7R)-7-(4-chloro-3-fluorophenyl)-1,4-oxazepan-6-yl]methyl}-2-oxo-1,2-dihydropyridine-3-carboxylic acid hydrochloride, which exhibited peripheral NET-selective inhibition at tested doses in rats by oral administration, increased urethral resistance in a dose-dependent manner.
REFERNCES
(a) Ishichi, Y.; Yamada, M.; Kamei, T.; Fujimori, I.; Nakada, Y.; Yukawa, T.; Sakauchi, N.; Ohba, Y.; Tsukamoto, T. WO 2012/046882 A1, Apr 12, 2012.
(b) Fujimori, I.; Yukawa, T.; Kamei, T.; Nakada, Y.; Sakauchi, N.; Yamada, M.; Ohba, Y.; Takiguchi, M.; Kuno, M.; Kamo, I.; Nakagawa, H.; Hamada, T.; Igari, T.; Okuda, T.; Yamamoto, S.; Tsukamoto, T.; Ishichi, Y.; Ueno, H. Bioorg. Med. Chem. 2015, 23, 5000– 5014 DOI: 10.1016/j.bmc.2015.05.017
(c) Yukawa, T.; Fujimori, I.; Kamei, T.; Nakada, Y.; Sakauchi, N.; Yamada, M.; Ohba, Y.; Ueno, H.; Takiguchi, M.; Kuno, M.; Kamo, I.; Nakagawa, H.; Fujioka, Y.; Igari, T.; Ishichi, Y.; Tsukamoto, T. Bioorg. Med. Chem. 2016, 24, 3207– 3217 DOI: 10.1016/j.bmc.2016.05.038
(d) Yukawa, T.; Nakada, Y.; Sakauchi, N.; Kamei, T.; Yamada, M.; Ohba, Y.; Fujimori, I.; Ueno, H.; Takiguchi, M.; Kuno, M.; Kamo, I.; Nakagawa, H.; Fujioka, Y.; Igari, T.; Ishichi, Y.; Tsukamoto, T. Bioorg. Med. Chem. 2016, 24, 3716– 3726 DOI: 10.1016/j.bmc.2016.06.014
//////////////////1372185-97-1, 1372180-09-0, Peripherally selective, noradrenaline reuptake inhibitor, TAKEDA
O=C(O)C3=CC=CN(C[C@@H]1CNCCO[C@H]1c2ccc(Cl)c(F)c2)C3=O