
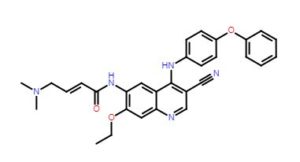
TL-487
CAS 1469746-55-1
2-Butenamide, N-[3-cyano-7-ethoxy-4-[(4-phenoxyphenyl)amino]-6-quinolinyl]-4-(dimethylamino)-, (2E)-
- Molecular Weight, 507.58, MF C30 H29 N5 O3
Teligene Inc(2E)-N-[3-Cyano-7-ethoxy-4-[(4-phenoxyphenyl)amino]-6-quinolinyl]-4-(dimethylamino)-2-butenamide
(E)-N-(3-cyano-7-ethoxy-4-((4-phenoxyphenyl)amino)quinolin-6-yl)-4-(dimethylamino)but-2-enamide
Maleate in anhydrous or monohydrate CAS, 2326561-36-6, AND 2326561-38-8 form are BTK and HER-2 kinase inhibitor useful for treating cancer
Useful for treating breast cancer, ovary cancer and colon cancer. are BTK and HER-2 kinase inhibitor useful for treating cancer.
Anticancer protein kinase inhibitor
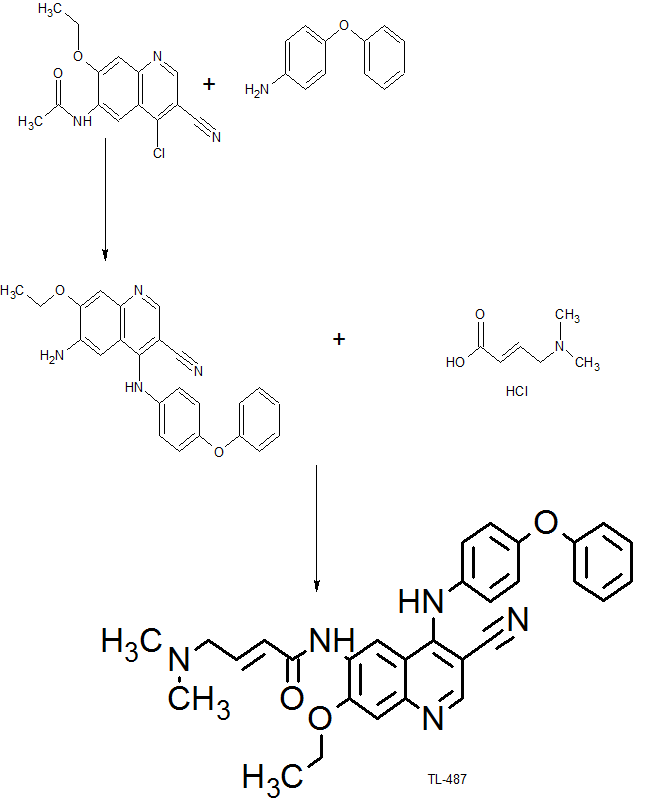
The compound was originally claimed in WO2013152135 , and may provide the structure of TL-487 , a small molecule inhibitor to HERs, being investigated by Teligene for the treatment of breast cancer; in July 2016, the company intended to develop the product as a class 1.1 chemical drug in China.
PATENT
US 20150057312
PATENT
WO2013152135
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2013152135&tab=PCTDESCRIPTION&queryString=%28ET%2Fkinase%29+&recNum=8&maxRec=4574
PATENT
WO-2019096327
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019096327&redirectedID=true
Novel crystalline maleate salt of (E)-N-(3-cyano-7-ethoxy-4-((4-phenoxyphenyl)amino)quinolin-6-yl)-4-(dimethylamino)but-2-enamide (first disclosed in WO2013152135) and its hydrates (monohydrate) and anhydrates, process for its preparation, composition comprising it and its use for treating cancers such as breast cancer, ovary cancer, colon cancer, prostate cancer, kidney cancer, bladder cancer, stomach cancer, lung cancer, mantle cell lymphoma and multiple myeloma are claimed. The compound is disclosed to be an irreversible inhibitor to BTK and Her-2 (also known as Erb-2 or neu).
(E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide is mentioned in WO2013152135 and corresponds to the compound of the Formula I:
Compounds derived from 3-cyanoquinoline have been shown to have anti-tumor activity, which may make them useful as chemotherapeutic agents in treating various cancers, including but not limited to, pancreatic cancer, melanoma, lymphatic cancer, parotid tumors, Barrett’s esophagus, esophageal carcinomas, head and neck tumors, ovarian cancer, breast cancer, epidermoid tumors, cancers of major organs, such as kidney, bladder, larynx, stomach, and lung, colonic polyps and colorectal cancer and prostate cancer. Examples of compounds derived from 3-cyanoquinoline are disclosed and shown to possess anti-tumor activity in many literatures. One limitation of certain 3-cyanoquinoline compounds is that they are not water soluble in a free base form.
The crystalline form of a particular drug as a salt, a hydrate and/or any polymorph thereof is often one important determinant of the drug’s ease of preparation, stability, water solubility, storage stability, ease of formulation and in-vivo pharmacology. It is possible that one crystalline form is preferable over another where certain aspects such as ease of preparation, stability, water solubility and/or superior pharmacokinetics are deemed to be critical. Crystalline forms of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide salts that possess a higher degree of water solubility than the free base but are stable fulfill an unmet need for stable, crystalline, water-solubl
Example 1. (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide sulfate
95%ethanol (4.0 ml) was added to (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide (500 mg, 0.99 mmol, 1.0 eq) , followed sulfuric acid (101.9 mg, 1.04 mmol, 1.05 eq) in 95%ethanol (1.0 ml) was added dropwise to the reaction mixture. Then an amount of precipitate was founded. Another 95% (60 ml) was added to the reaction mixture and the reaction mixture was heated to 70℃. Filtered and the filtrate was heated to 70℃ again. Then the reaction mixture was cooled to room temperature and The reaction mixture was crystallized at -10℃ for 41.5h. Filtered the precipitated solid and dried at 40℃ under vacuum for 1 hour to get the title compound (260 mg) as a yellow solid.
X-ray detection shows an amorphous structure to the compound as FIG. 9.
Example 2. Synthesis of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide hydrochloride
95%ethanol (5.0 ml) was added to (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide (500 mg, 0.99 mmol, 1.0 eq) , followed hydrochloric acid (38.0 mg, 1.04 mmol, 1.05 eq) in 95%ethanol (1.0 ml) was added dropwise to the reaction mixture. The reaction mixture was heated to 70℃. Filtered and the filtrate was crystallized under -10℃ for 44.5h. Filtered the precipitated solid and dried at 40℃ under vacuum for 1 hour to get the title compound (96 mg) as a yellow solid.
X-ray detection shows an amorphous structure to the compound in FIG. 6.
Example 3. Synthesis of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide malate
(E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide (500 mg, 0.99 mmol, 1.0 eq) , L-malic acid (139.4 mg, 1.04 mmol, 1.05 eq) and 95%ethanol (5.0 ml) was added to a 50 ml round-bottom flask. The reaction mixture was heated to 70℃. Filtered and the filtrate was crystallized under -10℃ for 45.5h. A little of precipitate was founded and then the reaction mixture was evaporated under vacuum at 40℃ to give the target (370 mg) as a yellow solid.
X-ray detection shows an amorphous structure to the compound in FIG. 8
Example 4: synthesis of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide citrate
To a solution of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide (500 mg, 0.99 mmol, 1.0 eq) , citric acid (198.8 mg, 1.04 mmol, 1.05 eq) and 95%ethanol (5.0 ml) . The reaction mixture was heated to 70℃. Filtered and the filtrate was crystallized under -10℃ for 45h. A little of precipitate was founded and then the reaction mixture was evaporated under vacuum at 40℃ to give the target compound (610 mg) as a yellow solid.
X-ray detection shows an crystalline structure to the compound in FIG. 7.
Example 5: Preparation of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide maleate monohydrate.
(E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide free base (0.091 kg) is rinsed with a 10%solution of USP purified water in n-propanol (0.082 kg, 0.10 L) followed by the addition of water: n-propanol solution (0.74 kg, 0.90 L) . Maleic acid is added (1.01 equiv) and the mixture is rinsed with 10%water: n-propanol (0.082 kg, 0.10 L) . The mixture is quickly heated to 50-60 ℃ and held for a minimum of 15 min. until a solution is obtained. The hot solution is clarified through a pre-heated 50-60 ℃, 0.2 Mm filter cartridge and the filtrates are collected in a preheated 45-55℃, 2 L multi-neck flask. The filter cartridge is rinsed through with 10%water: n-propanol pre-heated to 45-55 ℃ (0.082 kg, 0.10 L) . The solution is cooled over at least one hour to 40 ℃ and held at that temperature for 12 hours then cooled to room temperature (25 ℃) over a minimum of four hours and held at that temperature for at least two hours. The mixture is filtered on a 12.5 cm diameter Buchner funnel for 5 min., then rinsed and washed with prefiltered10%water: n-propanol solution (2 x 0.12 kg, 2 x 0.15 L) . The cake is dammed and suction maintained until dripping essentially stops, about 1 h.
Example 6: The product from Example 1 is dried (50 ℃, 10 mm Hg, 24 h) to give crystalline, anhydrous (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide maleate.
Example 7: Preparation of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide maleate monohydrate.
To a solution of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide (38.0 g, 75.0 mmol, 1.0 eq) and n-propanol/H 2O (380 ml, V: V=9: 1) . maleic acid (8.7 g, 75.0 mmol, 1.0 eq) in n-propanol/H 2O (76 ml, V: V=9: 1) was added to the reaction mixture. An amount of precipitate was founded, then the reaction mixturewas heated to 65 ℃. The solid was dissolved completely, then the reaction mixture was cooled to room temperature and stand for 20 hours. Filtered and filtrate was evaporated under vacuum to get the crude product.
The crude product (14.0 g) was recrystallized in n-propanol/H 2O (240 ml, V: V=9: 1) at 70℃. The solid was dissolved completely, then the reaction mixture was cooled to room temperature and stand for 20.5 hours. Filtered and wash the cake with n-propanol/H 2O (20 ml, V: V=9: 1) to get target product (12.9 g, wet) .
Example 8: crystalline, anhydrous (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide maleate.
To a solution of (E) -N- (3-cyano-7-ethoxy-4- ( (4-phenoxyphenyl) amino) quinolin-6-yl) -4- (dimethylamino) but-2-enamide (21.5 g, 42.4 mmol, 1.0 eq) and ethanol (300 ml) . maleic acid (5.2 g, 44.8 mmol, 1.05 eq) was added to the reaction mixture. An amount of precipitate was founded, then the reaction mixture was heated to 70 ℃. Another ethanol (1980 ml) was added to the reaction mixture in several times and the reaction temperature was keep at 70 ℃. Filtered and filtrate was cooled to room temperature, stop stirring and stand for 16-20 hours. Filtered and the solid was dried at room temperature for 24 hours to get the title compound.
///////////////TL-487, PRECLINICAL, CHINA, breast cancer, ovary cancer, olon cancer, BTK, HER-2 kinase inhibitor,
CN(C)C\C=C\C(=O)Nc3cc4c(Nc2ccc(Oc1ccccc1)cc2)c(cnc4cc3OCC)C#N
















