>>von Willebrand factor<<<
MIPARFAGVLLALALILPGTLCAEGTRGRSSTARCSLFGSDFVNTFDGSMYSFAGYCSYL
AGGCQKRSFSIIGDFQNGKRVSLSVYLGEFFDIHLFVNGTVTQGDQRVSMPYASKGLYLE
TEAGYYKLSGEAYGFVARIDGSGNFQVLLSDRYFNKTCGLCGNFNIFAEDDFMTQEGTLT
SDPYDFANSWALSSGEQWCERASPPSSSCNISSGEMQKGLWEQCQLLKSTSVFARCHPLV
DPEPFVALCEKTLCECAGGLECACPALLEYARTCAQEGMVLYGWTDHSACSPVCPAGMEY
RQCVSPCARTCQSLHINEMCQERCVDGCSCPEGQLLDEGLCVESTECPCVHSGKRYPPGT
SLSRDCNTCICRNSQWICSNEECPGECLVTGQSHFKSFDNRYFTFSGICQYLLARDCQDH
SFSIVIETVQCADDRDAVCTRSVTVRLPGLHNSLVKLKHGAGVAMDGQDIQLPLLKGDLR
IQHTVTASVRLSYGEDLQMDWDGRGRLLVKLSPVYAGKTCGLCGNYNGNQGDDFLTPSGL
AEPRVEDFGNAWKLHGDCQDLQKQHSDPCALNPRMTRFSEEACAVLTSPTFEACHRAVSP
LPYLRNCRYDVCSCSDGRECLCGALASYAAACAGRGVRVAWREPGRCELNCPKGQVYLQC
GTPCNLTCRSLSYPDEECNEACLEGCFCPPGLYMDERGDCVPKAQCPCYYDGEIFQPEDI
FSDHHTMCYCEDGFMHCTMSGVPGSLLPDAVLSSPLSHRSKRSLSCRPPMVKLVCPADNL
RAEGLECTKTCQNYDLECMSMGCVSGCLCPPGMVRHENRCVALERCPCFHQGKEYAPGET
VKIGCNTCVCRDRKWNCTDHVCDATCSTIGMAHYLTFDGLKYLFPGECQYVLVQDYCGSN
PGTFRILVGNKGCSHPSVKCKKRVTILVEGGEIELFDGEVNVKRPMKDETHFEVVESGRY
IILLLGKALSVVWDRHLSISVVLKQTYQEKVCGLCGNFDGIQNNDLTSSNLQVEEDPVDF
GNSWKVSSQCADTRKVPLDSSPATCHNNIMKQTMVDSSCRILTSDVFQDCNKLVDPEPYL
DVCIYDTCSCESIGDCACFCDTIAAYAHVCAQHGKVVTWRTATLCPQSCEERNLRENGYE
CEWRYNSCAPACQVTCQHPEPLACPVQCVEGCHAHCPPGKILDELLQTCVDPEDCPVCEV
AGRRFASGKKVTLNPSDPEHCQICHCDVVNLTCEACQEPGGLVVPPTDAPVSPTTLYVED
ISEPPLHDFYCSRLLDLVFLLDGSSRLSEAEFEVLKAFVVDMMERLRISQKWVRVAVVEY
HDGSHAYIGLKDRKRPSELRRIASQVKYAGSQVASTSEVLKYTLFQIFSKIDRPEASRIA
LLLMASQEPQRMSRNFVRYVQGLKKKKVIVIPVGIGPHANLKQIRLIEKQAPENKAFVLS
SVDELEQQRDEIVSYLCDLAPEAPPPTLPPHMAQVTVGPGLLGVSTLGPKRNSMVLDVAF
VLEGSDKIGEADFNRSKEFMEEVIQRMDVGQDSIHVTVLQYSYMVTVEYPFSEAQSKGDI
LQRVREIRYQGGNRTNTGLALRYLSDHSFLVSQGDREQAPNLVYMVTGNPASDEIKRLPG
DIQVVPIGVGPNANVQELERIGWPNAPILIQDFETLPREAPDLVLQRCCSGEGLQIPTLS
PAPDCSQPLDVILLLDGSSSFPASYFDEMKSFAKAFISKANIGPRLTQVSVLQYGSITTI
DVPWNVVPEKAHLLSLVDVMQREGGPSQIGDALGFAVRYLTSEMHGARPGASKAVVILVT
DVSVDSVDAAADAARSNRVTVFPIGIGDRYDAAQLRILAGPAGDSNVVKLQRIEDLPTMV
TLGNSFLHKLCSGFVRICMDEDGNEKRPGDVWTLPDQCHTVTCQPDGQTLLKSHRVNCDR
GLRPSCPNSQSPVKVEETCGCRWTCPCVCTGSSTRHIVTFDGQNFKLTGSCSYVLFQNKE
QDLEVILHNGACSPGARQGCMKSIEVKHSALSVELHSDMEVTVNGRLVSVPYVGGNMEVN
VYGAIMHEVRFNHLGHIFTFTPQNNEFQLQLSPKTFASKTYGLCGICDENGANDFMLRDG
TVTTDWKTLVQEWTVQRPGQTCQPILEEQCLVPDSSHCQVLLLPLFAECHKVLAPATFYA
ICQQDSCHQEQVCEVIASYAHLCRTNGVCVDWRTPDFCAMSCPPSLVYNHCEHGCPRHCD
GNVSSCGDHPSEGCFCPPDKVMLEGSCVPEEACTQCIGEDGVQHQFLEAWVPDHQPCQIC
TCLSGRKVNCTTQPCPTAKAPTCGLCEVARLRQNADQCCPEYECVCDPVSCDLPPVPHCE
RGLQPTLTNPGECRPNFTCACRKEECKRVSPPSCPPHRLPTLRKTQCCDEYECACNCVNS
TVSCPLGYLASTATNDCGCTTTTCLPDKVCVHRSTIYPVGQFWEEGCDVCTCTDMEDAVM
GLRVAQCSQKPCEDSCRSGFTYVLHEGECCGRCLPSACEVVTGSPRGDSQSSWKSVGSQW
ASPENPCLINECVRVKEEVFIQQRNVSCPQLEVPVCPSGFQLSCKTSACCPSCRCERMEA
CMLNGTVIGPGKTVMIDVCTTCRCMVQVGVISGFKLECRKTTCNPCPLGYKEENNTGECC
GRCLPTACTIQLRGGQIMTLKRDETLQDGCDTHFCKVNERGEYFWEKRVTGCPPFDEHKC
LAEGGKIMKIPGTCCDTCEEPECNDITARLQYVKVGSCKSEVEVDIHYCQGKCASKAMYS
IDINDVQDQCSCCSPTRTEPMQVALHCTNGSVVYHEVLNAMECKCSPRKCSK
Vonicog alfa
ボニコグアルファ (遺伝子組換え) ;
フォン・ヴィレブランド因子;
| Formula |
C9712H15373N2737O3032S210
|
|---|---|
| CAS |
109319-16-6
|
| Mol weight |
225723.1487
|
JAPAN 2020, APPROVED 2020/3/25, VONVENDI
Anticoagulant, Hemostatic, Replenisher (von Willebrand factor)
Active Substance
General information Recombinant von Willebrand Factor (rVWF) is co-expressed with recombinant Factor VIII (rFVIII) in Chinese hamster ovary (CHO) cells as part of the ADVATE (Centrally authorised product) manufacturing process. The rVWF protein is separated from the FVIII and further purified.
Structural formula
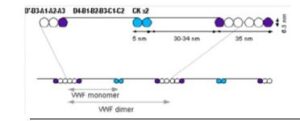
Vonicog alfa is expressed as a 2813 amino acid pro-VWF molecule. The pro-VWF is composed of A, B, C and D repeats, which contain various functional domains that have been identified. The mature VWF monomer is a 2050 amino acid protein. Every monomer contains a number of specific domains with a specific function. Elements of note are: • The D’/D3 domain, which binds to Factor VIII • The A1 domain, which binds to: Platelet gp1b-receptor, Heparin, Collagen • The A3 domain, which binds to collagen • The C1 domain, in which the RGD domain binds to platelet integrin αIIbβ3 when this is activated • The “cysteine knot” domain Monomers of pro-VWF are subsequently N-glycosylated, arranged into dimers via a C-terminal disulfide bond in the endoplasmic reticulum and into multimers by crosslinking of N-terminal cysteine residues via disulfide bonds.
Figure 1. Structure of Von Willebrand Factor Monomer/Dimer
After reduction of disulfide bonds in electrophoretic analysis, rVWF appears as a single predominant band having an apparent molecular weight of approximately 260 kDa. In low resolution agarose gel electrophoresis, rVWF shows a characteristic ladder of bands also known as multimers. In this analysis, rVWF contains as many distinct bands as VWF detectable in normal human plasma or VWF isolated from human plasma but in addition, has a zone with unresolved bands in the ultra-high molecular weight range. Highresolution electrophoresis shows a single band for all multimer levels without any satellite bands, as rVWF has never been exposed to ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) cleavage.

Vonicog alfa, sold under the brand names Vonvendi and Veyvondi, is a medicine used to control bleeding in adults with von Willebrand disease (an inherited bleeding disorder).[5][4][6] It is a recombinant von Willebrand factor.[5][4]
The most common adverse reactions are generalized itching, vomiting, nausea, dizziness, and vertigo.[5]
Vonicog alfa should not be used in the treatment of Hemophilia A.[4]
In the UK it is available only via a named patient access program.[7]
Vonicog alfa was approved for medical use in the United States in December 2015, in the European Union in August 2018, and in Australia in April 2020.[3][5][4][8] It was granted orphan drug designations in both the United States and the European Union.[4][1]
Medical uses
Vonicog alfa is indicated in adults with von Willebrand Disease (VWD), when desmopressin (DDAVP) treatment alone is ineffective or not indicated for the
Adverse effects
The following side effects may occur during treatment with vonicog alfa: hypersensitivity (allergic) reactions, thromboembolic events (problems due to the formation of blood clots in the blood vessels), development of inhibitors (antibodies) against von Willebrand factor, causing the medicine to stop working and resulting in a loss of bleeding control.[4] The most common side effects with vonicog alfa (which may affect up to 1 in 10 patients) are dizziness, vertigo (a spinning sensation), dysgeusia (taste disturbances), tremor, rapid heartbeat, deep venous thrombosis (blood clot in a deep vein, usually in the leg), hypertension (high blood pressure), hot flush, vomiting, nausea (feeling sick), pruritus (itching), chest discomfort, sensations like numbness, tingling, pins and needles at the site of infusion, and an abnormal reading on the electrocardiogram (ECG).[4]
References
- ^ Jump up to:a b c “Veyvondi Australian prescription medicine decision summary”. Therapeutic Goods Administration (TGA). 29 April 2020. Retrieved 16 August 2020.
- ^ “Vonvendi 650 IU powder and solvent for solution for injection – Summary of Product Characteristics (SmPC)”. (emc). 7 May 2020. Retrieved 16 August 2020.
- ^ Jump up to:a b “Vonvendi”. U.S. Food and Drug Administration (FDA). 9 May 2018. Archived from the original on 23 April 2019. Retrieved 15 April 2020.
- ^ Jump up to:a b c d e f g h i j “Veyvondi EPAR”. European Medicines Agency (EMA). 20 September 2018. Retrieved 27 March 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Jump up to:a b c d “Vonvendi (von willebrand factor- recombinant kit”. DailyMed. 13 February 2019. Retrieved 27 March 2020.
- ^ “Veyvondi-epar product information” (PDF). European Medicines Agency.
- ^ “Vonicog alfa”. Specialist Pharmacy Service. 15 January 2020. Retrieved 27 March 2020.
- ^ “Vonvendi”. U.S. Food and Drug Administration (FDA). 13 April 2018. STN: 125577. Retrieved 27 March 2020.
Further reading
- AusPAR: Vonicog alfa. Therapeutic Goods Administration (TGA) (Report). October 2020.
External links
- “Vonicog alfa”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Trade names | Vonvendi, Veyvondi |
| Other names | BAX-111 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| Drug class | Hemostatic |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C9712H15373N2737O3032S210 |
| Molar mass | 225725.54 g·mol−1 |
General References
- Singal M, Kouides PA: Recombinant von Willebrand factor: a first-of-its-kind product for von Willebrand disease. Drugs Today (Barc). 2016 Dec;52(12):653-664. doi: 10.1358/dot.2016.52.12.2570978. [PubMed:28276537]
- Brown R: Recombinant von Willebrand factor for severe gastrointestinal bleeding unresponsive to other treatments in a patient with type 2A von Willebrand disease: a case report. Blood Coagul Fibrinolysis. 2017 Oct;28(7):570-575. doi: 10.1097/MBC.0000000000000632. [PubMed:28379876]
- Gill JC, Castaman G, Windyga J, Kouides P, Ragni M, Leebeek FW, Obermann-Slupetzky O, Chapman M, Fritsch S, Pavlova BG, Presch I, Ewenstein B: Hemostatic efficacy, safety, and pharmacokinetics of a recombinant von Willebrand factor in severe von Willebrand disease. Blood. 2015 Oct 22;126(17):2038-46. doi: 10.1182/blood-2015-02-629873. Epub 2015 Aug 3. [PubMed:26239086]
- Lenting PJ, Christophe OD, Denis CV: von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015 Mar 26;125(13):2019-28. doi: 10.1182/blood-2014-06-528406. Epub 2015 Feb 23. [PubMed:25712991]
- Chung MC, Popova TG, Jorgensen SC, Dong L, Chandhoke V, Bailey CL, Popov SG: Degradation of circulating von Willebrand factor and its regulator ADAMTS13 implicates secreted Bacillus anthracis metalloproteases in anthrax consumptive coagulopathy. J Biol Chem. 2008 Apr 11;283(15):9531-42. doi: 10.1074/jbc.M705871200. Epub 2008 Feb 8. [PubMed:18263586]
- Boston Children’s Hospital [Link]
- EMA [Link]
- FDA application [Link]
- National Institute for Health Research [Link]
- Hemophilia [Link]
////////Vonicog alfa, JAPAN 2020, APPROVALS 2020,, VONVENDI, BAX 111,