WO 2009133389


IV V VI II
Example 1:
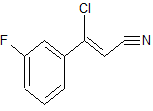
Synthesis of (Z)-3-Chloro-3-(3-fluorophenyl)-acrylonitrile from 3′-Fluoroacetophenone.

To a solution of 3′-fluoroacetophenone (80.0 g, 0.579 mol) in 7V,N-dimethyl formamide (560 ml) at about 400C was added phosphoryl chloride (92.50 ml, 1.01 mol) dropwise, maintaining the temperature at about 39-410C during the addition. The resulting reaction mixture was stirred at about 400C overnight before sampling for conversion to 2 by HPLC.
To the resulting reaction mixture was added a solution of hydroxylamine hydrochloride (45.17 g, 0.637 mol) in 7V,N-dimethyl formamide (240 ml) dropwise, maintaining the temperature at about 39-45°C during the addition, followed by a line -wash of JV,Λ/-dimethyl formamide (40 ml). After stirring at about 400C for 15 min, the reaction mixture was sampled for conversion to 4 before cooling to about 15-200C and addition of water (800 ml) dropwise, maintaining the temperature between about 17 to about 210C. The reaction mixture was then cooled to about 5°C and held at this temperature for a further 20 min before filtration of the solid, displacement washing with two separate portions of water (2 x 240 ml) and drying at about 400C overnight to afford the title compound as a pale yellow solid (74.24 g, 71% yield).
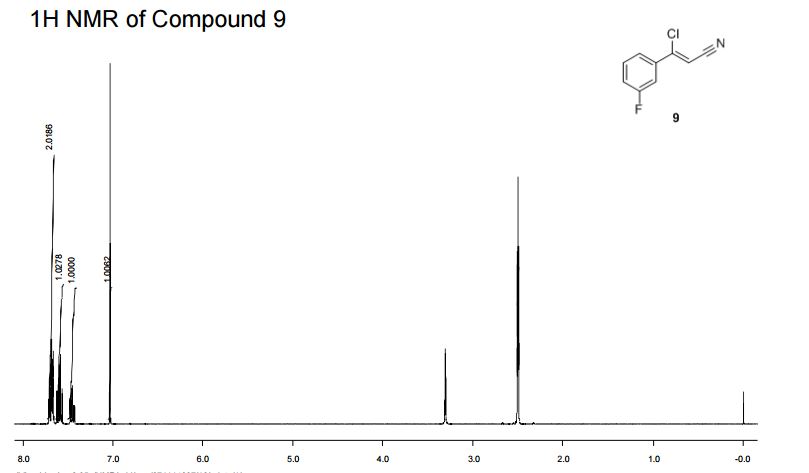
IH NMR (400MHz, DMSO-d6) δ: 7.72-7.65 (m, 2H), 7.63-7.56 (m, IH), 7.49-7.42 (m, IH),
7.03 (s, IH).
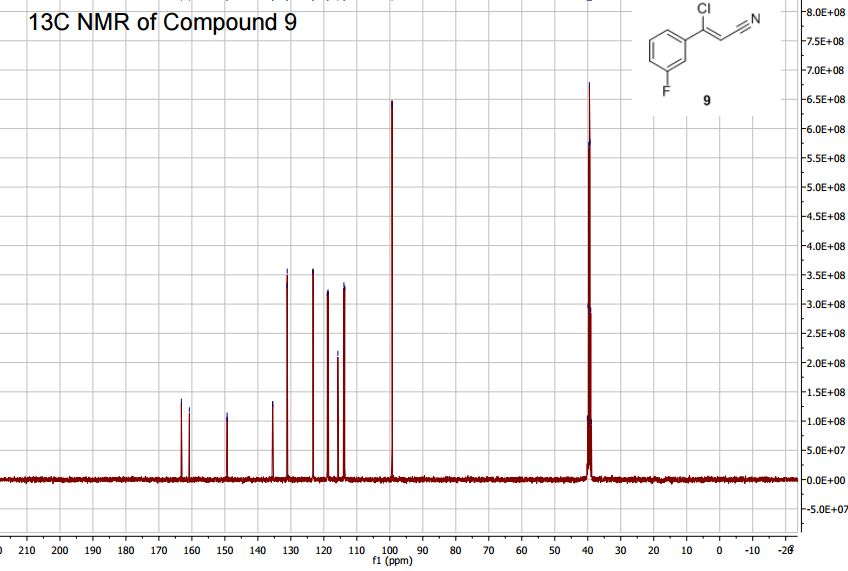
13C NMR (400MHz, DMSO-d6) δ: 162.0 (d, J = 245 Hz), 149.3 (d, J = 3 Hz), 135.6 (d, J= 8
Hz), 131.1 (d, J = 9 Hz), 123.3 (d, J = 3 Hz), 118.8 (d, J = 21 Hz), 115.8, 113.8 (d, J = 24 Hz),
89.3.
paper
(Z)-3-Chloro-3-(3′-fluorophenyl)-acrylonitrile (9)