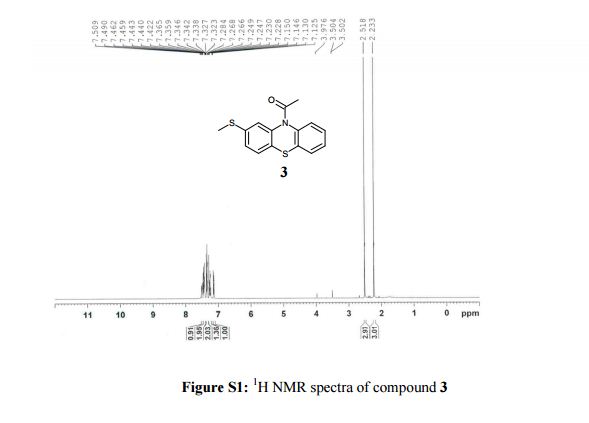


1-[2-(methylsulfanyl)-10H-phenothiazin-10-yl]ethanone (3): Off-white solid, yield. 93% (218 g),
m. p. 223-226 °C.
1H NMR (400 MHz, CDCl3, δ/ppm): 7.49 (d, 1H, arom H, J = 7.6 Hz), 7.46-7.42 (m, 2H, arom H), 7.36-7.32 (m, 2H, arom H), 7.28-7.22 (m, 1H, arom H), 7.13 (dd, 1H, arom H, J = 8.0 Hz and 1.6 Hz), 2.51 (s, 3H, -SCH3), 2.23 (s, 3H, -COCH3).
13C NMR (100 MHz, DMSO-d6, δ/ppm): 168.36, 139.19, 138.52, 137.74, 132.05, 128.07, 127.97, 127.78, 127.44, 127.19, 126.94, 124.60, 124.51, 22.71, 14.88.
MS m/z (ESI): 288.04 (M+H)+.
An efficient, practical, and commercially viable manufacturing process was developed with ≥99.7% purity and 31% overall yield (including four chemical reactions and one recrystallization) for an active pharmaceutical ingredient, called Metopimazine (1), an antiemetic drug used to prevent emesis during chemotherapy. The development of two in situ, one-pot methods in the present synthetic route helped to improve the overall yield of 1 (31%) compared with earlier reports (<15%). For the first time, characterization data of API (1), intermediates, and also possible impurities are presented. The key process issues and challenges were addressed effectively and achieved successfully.
//////////////////////















