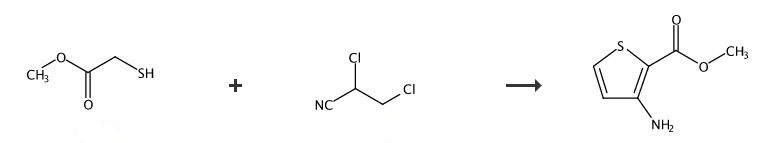
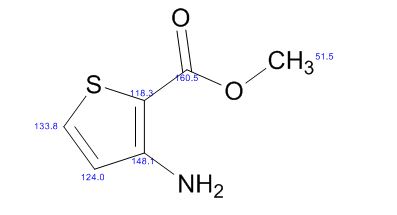
Methyl 3-amino-2-thiophenecarb
- Molecular Formula C6H7NO2S
- Average mass 157.190 Da
2-Thiophenecarboxylic acid, 3-amino-, methyl ester
Cas 22288-78-4
MW 157.19, MF C6 H7 N O2 S
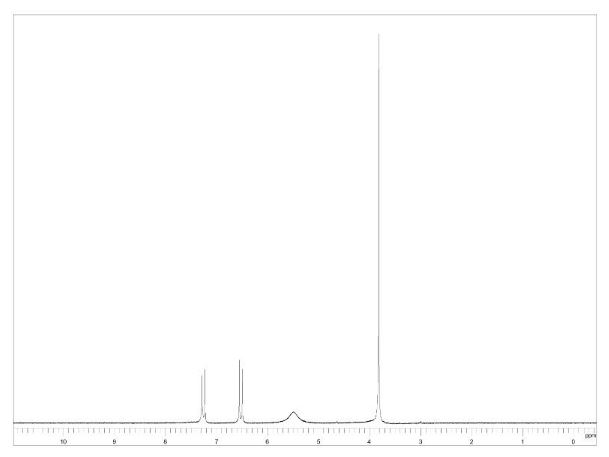
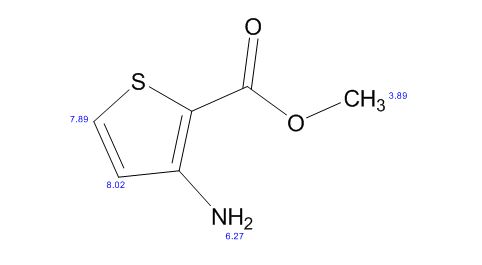
1H NMR CDCL3
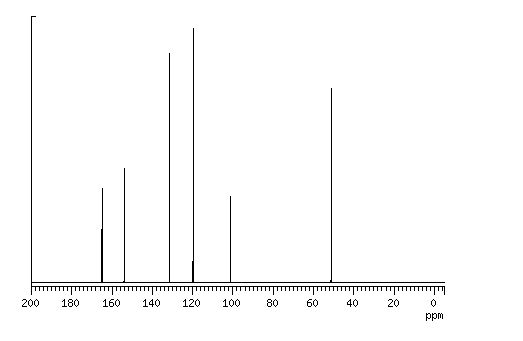
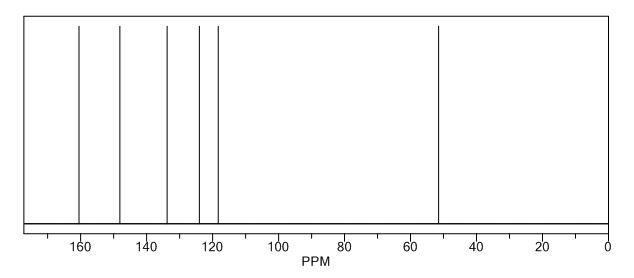
13C NMR CDCL3
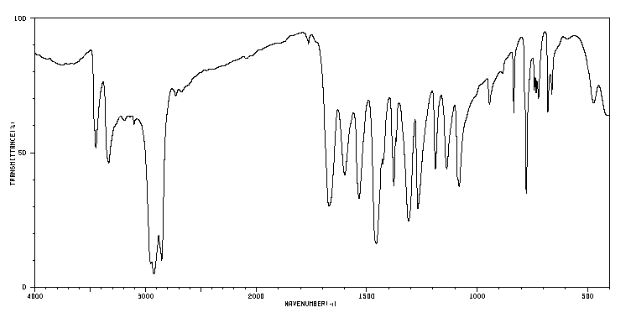
IR
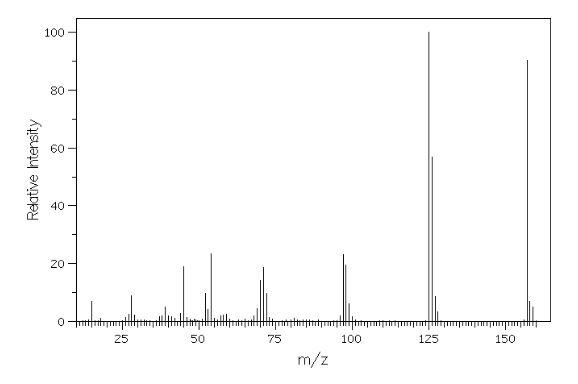
MASS
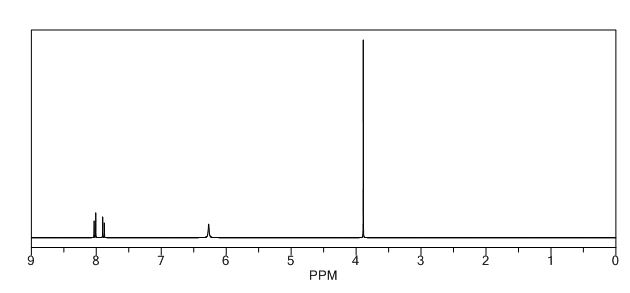
1H NMR PREDICT
13C NMR PREDICT
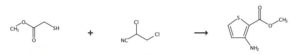
SYNTHESIS 1
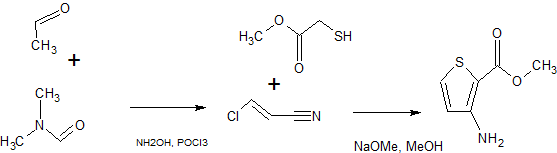
By Pokhodylo, Nazariy T. et alFrom Synthetic Communications, 44(7), 1002-1006; 2014
2-Propenenitrile, 3-chloro-, CAS : 871-29-4
SYNTHESIS 2
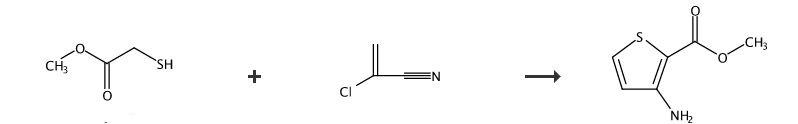
Rxn NaOMe, MeOH, 0°C
Methyl thioglycolate, CAS: 2365-48-2
C3 H2 Cl N
2-Propenenitrile, 2-chloro- OR 2-chloroacrylonitrile
920-37-6
By Denoyelle, Severine et alFrom European Journal of Organic Chemistry, 2015(32), 7146-7153; 2015
SYNTHESIS 2 B

Various Annellated Thieno [4,5]imidazo[2,1-b]thiazol-6-acetic Acids
By Schnatt, HeinzFrom No pp.; 1987
Step 1
3-Amino-2-thiophenecarboxylic acid methyl ester, hydrochloride (1):
202.5 g (3.75 mol) sodium methoxide was dissolved in 1000 mL absolute methanol and cooled to 20 degC. Subsequently, 159.2 g (1.50 mol) thioglycolic acid methyl ester was added dropwise within 10 minutes, while the temperature was increased to 26 degC. Then 131.3 g (1.50 mol) 2-chloroacrylonitrile was added dropwise within 2.5 hours, while the temperature was kept between 24 and 26 degC by water cooling. It was stirred for 30 minutes. By addition of 130 mL of glacial acetic acid, the mixture was neutralized (pH = 6), filtered over Hyflo and the solution was largely concentrated in vacuum. The residue was partitioned between 800 mL water and 400 mL ether and the aqueous phase was extracted three times with a total of 400 mL ether. The combined organic layers were dried over magnesium sulfate and filtered. Dry HCl gas was passed into this solution for 20 minutes. The precipitate was extracted three times with 100 mL dry ether and dried (50 degC/20 mbar). Yield: 172.3 g of yellowish crystals (60 % of theory). mp 128-129 degC. TLC: solvent: Bz:Et2O = 3:1, Rf = 0.7.
Step 2
3-Amino-2-thiophenecarboxylic acid methyl ester (2):
291.4 g (1.50 mol) 3-amino-2-thiophencarboxylic acid methyl ester, hydrochloride (1) were suspended in 875 mL methylene chloride and brought to pH = 9 with saturated sodium hydrogen carbonate solution. The reaction mixture was stirred until the completion of gas evolution. The phases were separated and the aqueous phase was extracted with a total of 500 mL methylene chloride. The combined organic layers were dried over sodium sulfate, filtered and evaporated. Yield: 221.7 pale yellow crystals (95 % of theory). mp 65-66 degC. LTC: solvent: Bz:Et2O = 3:1, Rf = 0.7.
SYNTHESIS 3
Me thioglycolate and 2-chloroacrylonitrile, and catalyzed by sodium methoxide.
2-chloroacrylonitrile:Me thioglycolate:sodium methoxide=1:1.2:3.3, reaction temp.=25-30°C and t=4h, and using sodium methoxide as Cat, the yield of 3-amino-2-thiophenecarboxylate was 88.2%
Guangdong Huagong, 39(7), 65, 42; 2012
Synthesis 4
Synthesis 2010(9): 1428-1430
DOI: 10.1055/s-0029-1218697
DOI: 10.1055/s-0029-1218697
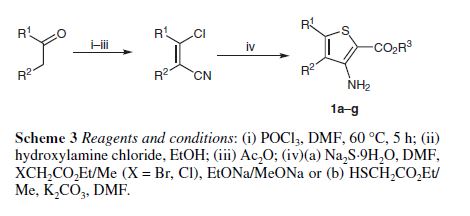
R = METHYL, THEN USE METHANOL AND SODIUM METHOXIDE
Paper
Bioorganic and Medicinal Chemistry, 2014, vol. 22, 7, p. 2113 – 2122
PAPER
Formation of stabilized organochlorogermylamines by the chelating organic substituent, 3-amino-2-methylthiophoate
Journal of Organometallic Chemistry (1991), 409, (1-2), 131-41.
Journal of Organometallic Chemistry (1991), 409, (1-2), 131-41.
PAPER
Structural modification of diketo acid portion in 1H-benzylindole derivatives HIV-1 integrase inhibitors
Heterocycles (2009), 78, (4), 947-959.
Heterocycles (2009), 78, (4), 947-959.
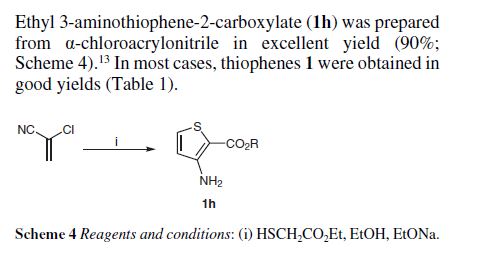
PATENT
GB 837086 1960
Treating 24.8 g. ClCH2CHClCN with 31.8 g. thioglycollic acid Me ester in the presence of 29.2 g. NaOMe and 280 cc. Et2O gave 72% Me ester of 3-aminothiophene-2-carboxylic acid (I), b0.1 100-2°, m. 65.5°;

SYNTHESIS
Synthesis of methyl 5-chloro-3-(methylaminosulfonyl)thiophene-2-carboxylate
Faming Zhuanli Shenqing Gongkai Shuomingshu (2008),
Faming Zhuanli Shenqing Gongkai Shuomingshu (2008),
NaOMe, MeOH, < 20°C; 30-60 min, < 20°C
10-15°C; 4 h, 10-15°C, ……….H2O, cooled
- “PhysProp” data were obtained from Syracuse Research Corporation of Syracuse, New York (US)
Bp 101 deg cent
///////