
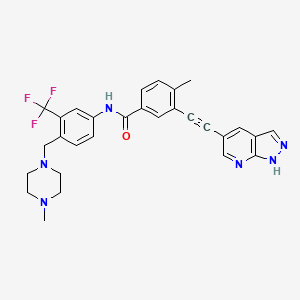
Olverembatinib
1257628-77-5- 3-((1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)benzamide
- HQP1351
- 4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]-3-[2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl]benzamide
- HQP1351 is under investigation in clinical trial NCT03883100 (A Pivotal Study of HQP1351 in Patients of Chronic Myeloid Leukemia in Accelerated Phase With T315I Mutation).
- 4-methyl-N-[4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl]-3-[2-(1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl]benzamide
- D-824
- GZD824
WeightAverage: 532.571
Monoisotopic: 532.219844002, Chemical FormulaC29H27F3N6O
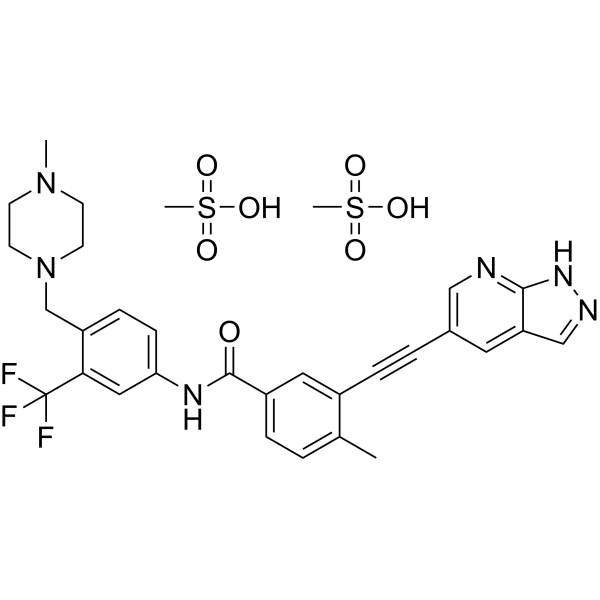
| Molecular Weight | 724.77 |
|---|---|
| Formula | C31H35F3N6O7S2 |
Olverembatinib (GZD824) dimesylate is a potent and orally active pan-Bcr-Abl inhibitor. Olverembatinib dimesylate potently inhibits a broad spectrum of Bcr-Abl mutants. Olverembatinib dimesylate strongly inhibits native Bcr-Abl and Bcr-AblT315I with IC50s of 0.34 nM and 0.68 nM, respectively. Olverembatinib dimesylate has antitumor activity. Olverembatinib (dimesylate) is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
Olverembatinib is a BCR-ABL1 tyrosine kinase inhibitor developed by Ascentage Pharma. In 2021, it was approved in China “for the treatment of adult patients with TKI-resistant chronic-phase CML (CML-CP) or accelerated-phase CML (CML-AP) harbouring the T315I mutation”.[1][2][3]
SYN
Ren, Xiaomei;Pan, Xiaofen;Zhang, Zhang;Wang, Deping;Lu, Xiaoyun;Li, Yupeng;Wen, Donghai;Long, Huoyou;Luo, Jinfeng;Feng, Yubing;Zhuang, Xiaoxi;Zhang, Fengxiang;Liu, Jianqi;Leng, Fang;Lang, Xingfen;Bai, Yang;She, Miaoqin;Tu, Zhengchao;Pan, Jingxuan;Ding, Ke [Journal of Medicinal Chemistry,2013,vol. 56,# 3,p. 879 – 894]
https://pubs.acs.org/doi/10.1021/jm301581y
PATENT
CN 114163434
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN355399053&_cid=P10-MDPKRT-75688-1
| Example |
| The following examples further illustrate but do not limit the present invention. It should be noted that those skilled in the art can make various modifications and improvements without departing from the inventive concept of the present invention, all of which are included in the scope of protection of the present invention. |
| The specific conditions not disclosed in the experimental methods of the following examples can be selected according to conventional methods and conditions, or according to the product instructions. |
| Unless otherwise specified, “room temperature” in the following examples refers to 20°C to 25°C. The term “h” used herein refers to hours. |
| Example 1 |
| Step 1: |
| |
| Under nitrogen, N-methylpyrrolidone (137.6 g) was heated to 30-35°C to obtain the compound of Formula 1 (14.4 g, 1.3 eq) and the compound of Formula 2 (19.14 g, 1 eq). Bis(triphenylphosphate)palladium dichloride (0.46 g, 0.01 eq) and cuprous iodide (0.113 g, 0.01 eq) were added sequentially. Triethylamine (9.45 g, 1.5 eq) was then added under nitrogen. The reaction mixture was heated to 65-75°C and maintained at this temperature for 2 hours. The reaction process was monitored by liquid chromatography-mass spectrometry. The reaction was terminated when the content of the compound of Formula 2 was ≤0.1%. After completion of the reaction, the reaction solution was cooled to 35-45°C and N-acetyl-L-cysteine (1 g, 0.1 eq) was added directly. The reaction was stirred for 4-5 hours. The resulting product was cooled to room temperature, precipitated with water, centrifuged, and washed with pure water to obtain a crude filter cake. The crude filter cake was vacuum-dried and then slurried with a mixture of ethyl acetate and n-heptane (5 mL of the mixed solvent, wherein the volume ratio of ethyl acetate to n-heptane was 1:1) at a rate of 5 mL per gram of crude filter cake. The resulting slurry was vacuum-dried to yield the compound of Formula 3 with a yield of 85.97% and a purity of 98.2%. |
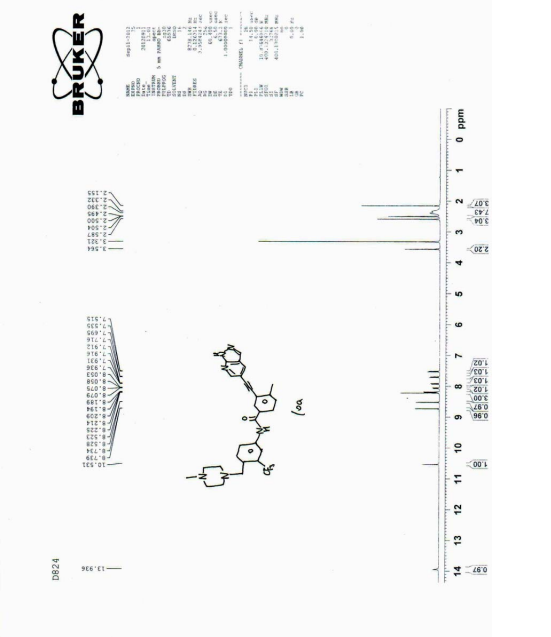
| The NMR data for the compound of Formula 3 are as follows : 1 H NMR (400 MHz, d-DMSO): δ ppm: 8.93 (1H, d, J = 2.0 Hz); 8.63 (1H, d, J = 2.0 Hz); 8.49 (1H, s); 8.11 (1H, d, J = 2.0 Hz); 7.92 (1H, dd, J = 1.6 Hz; J = 8.0 Hz); 7.52 (1H, d, J = 8.0 Hz); 3.88 (3H, s); 2.59 (3H, s); 1.65 (9H, s). |
| Step 2: |
| |
| Under nitrogen, methanol (160 g) and water (50 g) were sequentially added to the compound of formula 3 (20 g, 1.0 eq). The reaction system was stirred at reflux for 18 hours with process control. The resulting product was cooled to room temperature and filtered to obtain a filter cake (no drying required). Recrystallization was performed by adding 10 times the mass of the filter cake in methanol. The resulting mixture was stirred at 60-70°C for 8-10 hours, then cooled to 40-50°C and subjected to a gradient cooling process at a cooling rate of 5°C per 1 to 1.5 hours to slowly form a solid precipitate. The resulting mixture was filtered, the filter cake was washed with methanol, and vacuum dried to obtain the compound of formula 4 in a 91% yield and 99.7% purity. |
| The NMR data for the compound of Formula 4 are as follows : 1 H NMR (400 MHz, d-DMSO): δ ppm: 8.73 (1H, d, J = 2.0 Hz); 8.52 (1H, t, J = 2.0 Hz); 8.21 (1H, d, J = 2.0 Hz); 8.06 (1H, s); 7.86 (1H, dd, J1 = 2.0 Hz; J2 = 8.0 Hz); 7.49 (1H, dd, J1 = 1.6 Hz; J2 = 7.6 Hz); 3.86 (3H, s); 2.56 (3H, s). |
| Step 3: |
| |
| Under nitrogen, THF (448 mL), compound of formula 4 (29.1 g, 1 eq), and compound of formula 5 (24.6 g, 0.9 eq) were added, stirred, and cooled to -65°C to -60°C. At this temperature, potassium tert-butoxide (19 g x 3) was added in batches every 0.5 h. The reaction process was controlled by liquid phase detection. After 2 hours, the reaction temperature was raised to -5 to 0°C. The reaction solution was washed with purified water, stirred for 0.5-1 hour, washed with brine, and separated to obtain an organic phase. N-acetyl-L-cysteine (11.41 g, 0.7 eq) was added to the organic phase, stirred, washed with brine, neutralized, and concentrated under reduced pressure. The resulting filter cake was washed with purified water and made into a slurry. The resulting product was washed again with purified water and dried under vacuum to obtain compound of formula 6 with a yield of 88.2% and a purity of 98.6%. |
| The NMR data for the compound of formula 6 are as follows : 1 H NMR (400 MHz, d-DMSO): δ ppm: 10.53 (1H, s); 8.75 (d, J = 2.0); 8.53 (d, J = 2.4); 8.24 (1H, s); 8.23 (d, J = 2.4); 8.21 (d, J = 1.6); 8.09 (dd, J1 = 1.6; J2 = 8.4); 7.94 (dd, J1 = 2.0; J2 = 8.0); 7.71 (d, J = 8.8); 7.53 (d, J = 8.0); 3.56 (2H, s); 2.59 (3H, s); 2.34-2.35 (8H, m), 2.16 (3H, s). |
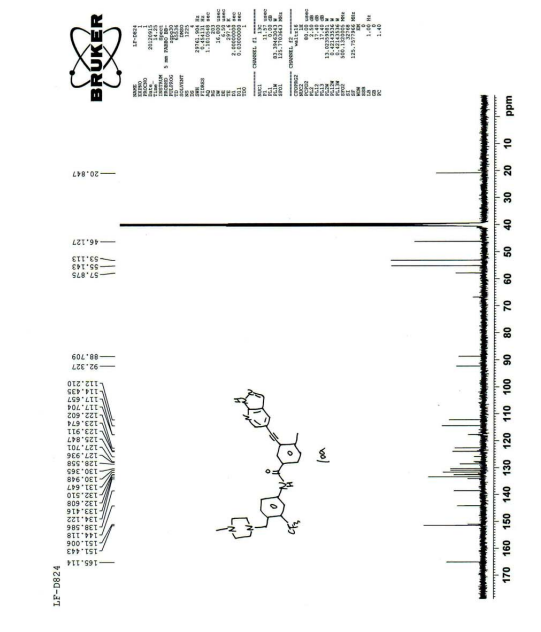
| Its carbon spectrum data are 13 C NMR (100 MHz, d-DMSO): δ ppm: 20.38, 45.65, 52.64, 54.67, 57.41, 88.26, 91.86, 111.76, 113.98, 117.19, 122.14, 123.43, 127.35 (q), 124.30 (q), 128.10, 129.89, 130.49, 131.15, 132.02, 132.13, 132.93, 133.66, 138.15, 143.65, 150.55, 164.64. |
PATENT
CN 101885722
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN84081329&_cid=P10-MDPKML-68458-1
| Example 23 |
| 3-((1H-pyrazolo[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1-substituted)methyl)3-(trifluoromethyl)phenyl)benzamide (D824) |
| (3-((1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-4-methyl-N-(4-((4-methylpiperazin-1-yl)m ethyl)-3-(trifluoromethyl)phenyl)benzamide) |

| The synthesis method is the same as in Example 1. |
| 1 HNMR (400MHz, d-DMSO), δ13.92 (s, 1H), 10.55 (s, 1H), 8.72 (d, J=2.0Hz, 1H), 8.52 (d, J=2.0Hz, 1H), 8.17 (m, 3H), 8.10 (d, J=8.0Hz, 1H), 7.92 (dd, J=8.0, 2.0Hz, 1H), 7.70 (d, J=8.8Hz, 1H), 7.53 (d, J=8.0Hz, 1H), 3.80 (s, 2H), 3.10 (brs, 8H), 2.71 (s, 3H), 2.57 (s, 3H). |
| MS(ESI), m/z: 533, (M + +H + ). |
SYN
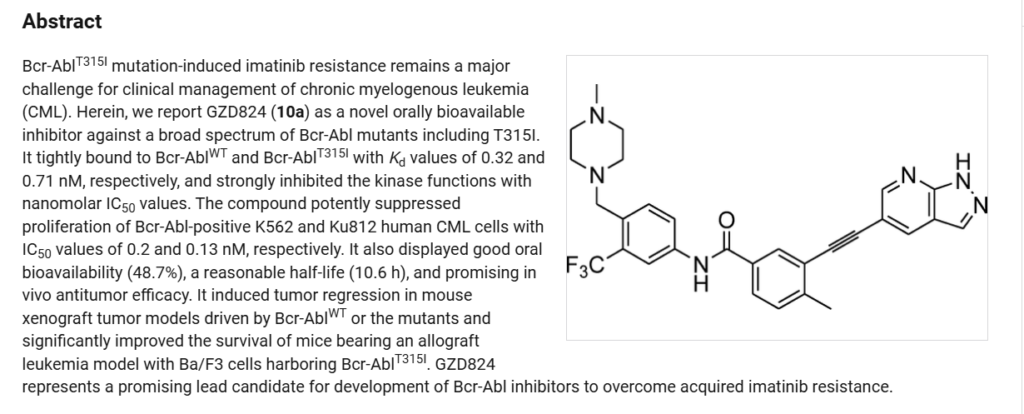
Olverembatinib(24) wasdeveloped by Ascentage Pharma as anorally available, third-generation
tyrosinekinase inhibitor (TKI) for the treatment of chronic myeloid leukemia (CML), acute myeloid leukemia, acute lymphoblastic leukemia (ALL), and solid tumors.167 It received its first approval inChina inNovember 2021 and was approved for use in adults with TKI-resistant CML chronicphaseandCML-acceleratephaseharboringtheT315I “gatekeeper” mutation.168 The current mainstay of CML
treatmentiscenteredaroundTKIs;however,resistancetoTKItherapy, often through BCR-ABL1 kinase domain point mutations, remains a challenge for early generation therapies.169Olverembatinibretainsitsefficacybyfunctioningasan ATP-bindingsiteinhibitorofwild-typeBCR-ABL1kinaseand broadly relatedmutants including T315I, which otherwise confers resistance against all first and second generation TKIs.168
Thesynthesisofolverembatinibhasbeenreportedinseveral patents,170−172 aswell as a journal article173 that details the divergentapproachtorelatedanalogues. Inarecentpatent,170 the synthesis of olverembatinib began with a Sonogashira coupling of commercially available alkyne 24.1 with
bromopyridine24.2toaffordester24.3in98%yield(Scheme43). Cleavage of the N-Boc group was accomplished by refluxingcarbamate24.3inaMeOHandwatermixturetogive pyrazole24.4 in91%yield. AfinalKOtBumediatedamide formation with aniline 24.5 resulted in the isolation of
olverembatinib(24) in88%yield.
(167) Dhillon, S. Olverembatinib: First approval. Drugs 2022, 82,
469−475.
(168) Braun, T. P.; Eide, C. A.; Druker, B. J. Response and resistance
to BCR-ABL1-targeted therapies. Cancer Cell 2020, 37, 530−542.
(169) Shoukier, M.; Kubiak, M.; Cortes, J. Review of new-generation
tyrosine kinase inhibitors for chronic myeloid leukemia. Curr. Oncol.
Rep. 2021, 23, 91.
(170) Wen, J.; Feng, J.; Wu, T.; Cai, M.; Teng, S. Preparation
method of alkynyl containing compound and its intermediate. China
Patent CN 114163434, 2022.
(171) Guo, M.; Wen, J.; Teng, S.; Wu, T.; Feng, J. Preparation of
(trifluoromethylphenyl)(pyrazolo[3,4-b]pyridinylethynyl)benzamide
derivative. China Patent CN 113292556, 2021.
(172) Ding, K.; Wang, D.; Pei, D.; Zhang, Z.; Shen, M.; Luo, K.;
Feng, Y. Heterocyclic alkynylbenzene derivatives as cancer cell line
inhibitors and their preparation, pharmaceutical compositions and use
in the treatment of cancer. China Patent CN 101885722, 2010.
(173) Ren, X.; Pan, X.; Zhang, Z.; Wang, D.; Lu, X.; Li, Y.; Wen, D.;
Long, H.; Luo, J.; Feng, Y.; et al. Identification of GZD824 as an
orally bioavailable inhibitor that targets phosphorylated and non
phosphorylated breakpoint cluster region−abelson (Bcr-Abl) kinase
and overcomes clinically acquired mutation-induced resistance against
imatinib. J. Med. Chem. 2013, 56, 879−894.




AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
References
- Dhillon, Sohita (March 2022). “Olverembatinib: First Approval”. Drugs. 82 (4): 469–475. doi:10.1007/s40265-022-01680-9. PMID 35195876. S2CID 247027755.
- Jiang, Qian; Li, Zongru; Qin, Yazhen; Li, Weiming; Xu, Na; Liu, Bingcheng; Zhang, Yanli; Meng, Li; Zhu, Huanling; Du, Xin; Chen, Suning; Liang, Yang; Hu, Yu; Liu, Xiaoli; Song, Yongping; Men, Lichuang; Chen, Zi; Niu, Qian; Wang, Hengbang; Lu, Ming; Yang, Dajun; Zhai, Yifan; Huang, Xiaojun (18 August 2022). “Olverembatinib (HQP1351), a well-tolerated and effective tyrosine kinase inhibitor for patients with T315I-mutated chronic myeloid leukemia: results of an open-label, multicenter phase 1/2 trial”. Journal of Hematology & Oncology. 15 (1): 113. doi:10.1186/s13045-022-01334-z. PMC 9389804. PMID 35982483.
- Jiang, Qian; Huang, Xiaojun; Chen, Zi; Niu, Qian; Shi, Dayu; Li, Zongru; Hou, Yue; Hu, Yu; Li, Weiming; Liu, Xiaoli; Xu, Na; Song, Yongping; Zhang, Yanli; Meng, Li; Hong, Zhenya; Liu, Bingcheng; Zeng, Shan; Men, Lichuang; Li, Yan; Chen, Suning; Xue, Mengxing; Zhu, Huanling; Li, He; Du, Xin; Lou, Jin; Zhang, Xiaohan; Liang, Yang; Dai, Yujun; Lu, Ming; Wang, Hengbang; Ji, Jiao; Yue, Changai; Yang, Dajun; Zhai, Yifan (5 November 2020). “Novel BCR-ABL1 Tyrosine Kinase Inhibitor (TKI) HQP1351 (Olverembatinib) Is Efficacious and Well Tolerated in Patients with T315I-Mutated Chronic Myeloid Leukemia (CML): Results of Pivotal (Phase II) Trials”. Blood. 136 (Supplement 1): 50–51. doi:10.1182/blood-2020-142142. S2CID 228875477.
| Clinical data | |
|---|---|
| Other names | GZD-824; GZD824 |
| Legal status | |
| Legal status | Investigational |
| Identifiers | |
| IUPAC name | |
| CAS Number | 1257628-77-5 |
| PubChem CID | 51038269 |
| IUPHAR/BPS | 10630 |
| DrugBank | DB16185 |
| ChemSpider | 29395146 |
| UNII | KV1M7Q3CBP |
| ChEMBL | ChEMBL2316582 |
| CompTox Dashboard (EPA) | DTXSID301352011 |
| Chemical and physical data | |
| Formula | C29H27F3N6O |
| Molar mass | 532.571 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
[1]. Ren X, Pan X, Zhang Z, Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem. 2013 Feb 14;56(3):879-94. [Content Brief]
//////////Olverembatinib, approvals 2021, china 2021, Ascentage Pharma, cancer, HQP1351, HQP 1351, D-824, D 824, KV1M7Q3CBP, GZD824














