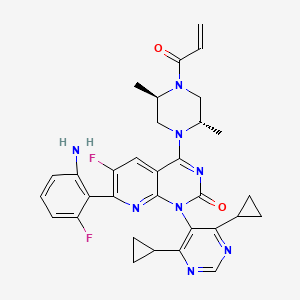
Garsorasib
Chemical Formula: C32H32F2N8O2
Exact Mass: 598.2616
Molecular Weight: 598.66
- CAS 2559761-14-5
- P491NE9G6Z
- D-1553
- 7-(2-amino-6-fluorophenyl)-1-(4,6-dicyclopropylpyrimidin-5-yl)-4-[(2S,5R)-2,5-dimethyl-4-prop-2-enoylpiperazin-1-yl]-6-fluoropyrido[2,3-d]pyrimidin-2-one
- 4-((2S,5R)-4-Acryloyl-2,5-dimethylpiperazin-1-yl)-7-(2-amino-6-fluorophenyl)-1-(4,6-dicyclopropylpyrimidin-5-yl)-6-fluoropyrido(2,3-d)pyrimidin-2(1H)-one
- Pyrido(2,3-d)pyrimidin-2(1H)-one, 7-(2-amino-6-fluorophenyl)-1-(4,6-dicyclopropyl-5-pyrimidinyl)-4-((2S,5R)-2,5-dimethyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-6-fluoro-
D 1553, Chia Tai Tianqing, CHINA 2024, APPROVALS 2024, Anfangning,
Garsorasib is an orally available inhibitor of the oncogenic KRAS substitution mutation, G12C, with potential antineoplastic activity. Upon oral administration, garsorasib selectively targets the KRAS G12C mutant and inhibits KRAS G12C mutant-dependent signaling. KRAS, a member of the RAS family of oncogenes, serves an important role in cell signaling, division and differentiation. Mutations of KRAS may induce constitutive signal transduction leading to tumor cell growth, proliferation, invasion, and metastasis.
PAT
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021120045&_cid=P11-MEJTS8-41135-1
Example 5. Preparation and Solid state characterization of Compound 2
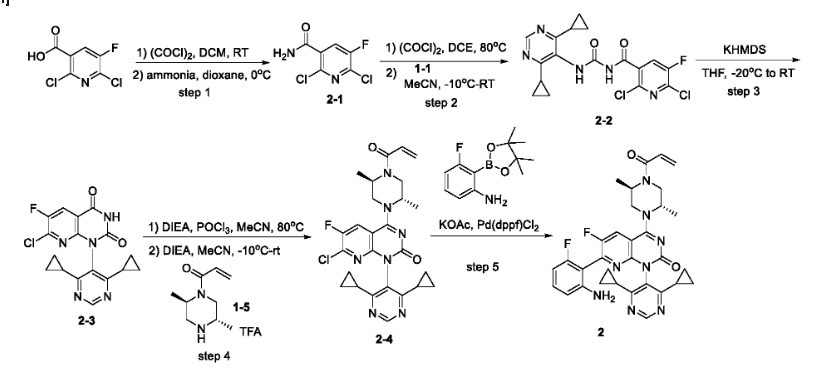
Step 1: To a mixture of 2, 6-dichloro-5-fluoronicotinic acid (23 g, 0.11 mol) in dichloromethane (300 mL) was added dimethylformamide (0.2 mL) . Oxalyl chloride (33 g, 0.26 mol) was then added slowly over 30 minutes at room temperature. The mixture was stirred at room temperature for an hour and then concentrated to give an oil which was dissolved in dioxane (50 mL) . The solution was added to ammonium hydroxide (150 mL) at 0℃ over 30 minutes. The resulting mixture was stirred at 0℃ for 30 minutes and then filtered. The filter cake was washed with cooled water (50 mL) and dried to afford 2-1.
[0183]
Step 2: A solution of 2-1 (11 g, 52.6 mmol) in 1, 2-dichloroethane (80 mL) was treated with oxalyl chloride (8.68 g, 68.4 mmol) . The mixture was stirred at 80℃ for 45 minutes and the reaction was concentrated. The residue was dissolved in acetonitrile (100 mL) , cooled to -10℃, and a solution of 1-1 (9.6 g, 55.2 mmol) in THF (30 mL) was added. The resulting mixture was stirred at room temperature for 2 hours. The solution was diluted with a sat. aqueous NaHCO 3solution and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and concentrated. The residue was purified by column chromatography on silica gel (petroleum ether to petroleum ether/ethyl acetate = 4/1) to afford 2-2.
[0184]
Step 3: To a stirred solution of 2-2 (7.9 g, 19.3 mmol) in THF (100 mL) at -20℃ was added KHMDS (38.6 mL, 1 M in THF, 38.6 mmol) . The resulting mixture was stirred at room temperature for 2 hours. The reaction was quenched with sat. aqueous NH 4Cl solution and extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate and concentrated. The residue was purified by flash column chromatography on silica gel (petroleum ether to petroleum ether/ethyl acetate = 2/1) to afford 2-3.
[0185]
Step 4: To a solution of 2-3 (746 mg, 2 mmol) and DIEA (387 mg, 3 mmol) in MeCN (20 mL) was added POCl 3(367 mg, 2.4 mmol) dropwise at room temperature. The resulting mixture was stirred at 80℃ for 45 minutes, followed by addition of DIEA (3.87 g, 30 mmol) and a solution of 1-5 (1.58 g, 4 mmol) in MeCN (10 mL) dropwise at -10℃. After stirring at room temperature for 1 hour, the reaction was quenched with ice-water and the mixture was extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate and concentrated. The residue was purified by flash column chromatography on silica gel (dichloromethane to dichloromethane/methanol = 10/1) to afford 2-4.
[0186]
Step 5: A mixture of 2-4 (8 mg, 0.15 mmol) , 3-fluoro-2- (4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolan-2-yl) aniline (42 mg, 0.18 mmol) , Pd (dppf) Cl 2(13 mg, 0.018 mmol) and KOAc (40 mg, 0.41 mmol) in dioxane (3 mL) /H 2O (1 drop) was stirred at 80℃ for 2 hours under nitrogen atmosphere. The mixture was diluted with water and extracted with ethyl acetate. The combined organic layers were dried over anhydrous Na 2SO 4and concentrated. The residue was purified by a Prep-HPLC (acetonitrile with 0.05%of TFA in water (30%to 65%) to afford 2. LCMS (ESI, m/z) : [M+H] += 599.1; HNMR (400 MHz, methanol-d 4, ppm) : δ 8.73 (s, 1H) , 8.26-8.22 (m, 1H) , 7.15-7.09 (m, 1H) , 6.84-6.74 (m, 1H) , 6.53 (d, J = 8.4 Hz, 1H) , 6.42-6.38 (m, 1H) , 6.30-6.24 (m, 1H) , 5.83-5.78 (m, 1H) , 5.01 (brs, 1H) , 4.91-4.83 (m, 1H) , 4.53-4.29 (m, 2H) , 3.96-3.89 (m, 1.5H) , 3.54-3.50 (m, 0.5H) , 1.82-1.75 (m, 1H) , 1.73-1.66 (m, 1H) , 1.47 (d, J = 6.8 Hz, 3H) , 1.37-1.27 (m, 3H) , 1.16-1.05 (m, 4H) , 1.03-0.97 (m, 2H) , 0.88-0.83 (m, 2H) . FNMR (376 MHz, methanol-d 4, ppm) : δ -114.9 (1F) , -125.6 (1F) .
[0187]
Compound 2 prepared via the above procedure was slurried in EtOAc, and filtered to provide Compound 2 in a crystalline form A. About 1.1%of residual EtOAc was detected by 1H-NMR, corresponding to weight loss at 120 –290 ℃ in TGA (FIG. 5B) . Two overlapped endothermic peaks were observed by DSC (FIG. 5B) . Compound 2 in Form A was heated to 250 ℃ and DSC profile of the residual solid was unchanged, suggesting the overlapped peak was due to melting with crystal form transformation. Thus, the starting material was an anhydrate.
[0188]
Form A was very soluble in DCM (> 92 mg/mL) and soluble (20 –33 mg/mL) in MeOH, butanone, THF, ACN and acetone. In other solvents, Form A was practically insoluble
SYN
CN112585129
https://patentscope.wipo.int/search/en/detail.jsf?docId=CN321747237&_cid=P11-MEJTN6-36089-1
SYN
European Journal of Medicinal Chemistry 291 (2025) 117643
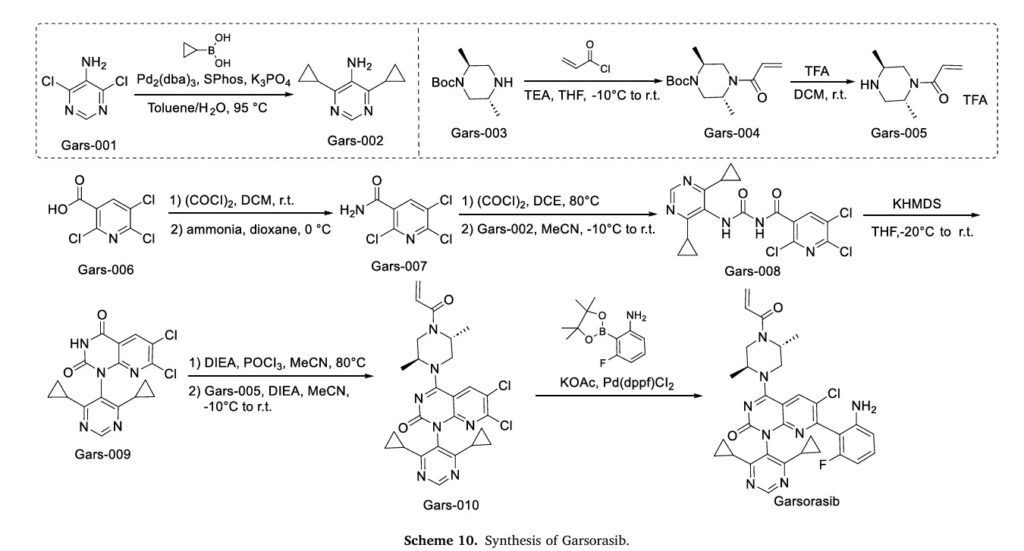
Garsorasib (D-1553), marketed as Anfangning, is an orally bioavailable KRAS G12C inhibitor jointly developed by InventisBio and Chia Tai Tianqing Pharmaceutical Group [40]. This compound is specifically engineered to target the KRAS G12C mutation, a prevalent oncogenic driver in multiple cancers, including NSCLC. In 2024, the NMPA granted conditional approval for Garsorasib to treat adult patients with advanced NSCLC harboring the KRAS G12C mutation, who have undergone at least one prior systemic therapy regimen [41]. Garsorasib exerts its pharmacological effects through selective and irreversible binding to the KRAS G12C mutant protein, thereby immobilizing it in an inactive GDP-bound conformation. This binding event effectively disrupts the activation of downstream signaling path
ways, including mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K), resulting in diminished tumor cell proliferation and survival. The clinical efficacy of Garsorasib has been
confirmed in a Phase II clinical trial (NCT05383898) involving patients with advanced NSCLC harboring the KRAS G12C mutation. The trial reported an ORR of 52.0 % and a DCR of 88.6 %. Additionally, the
median PFS was observed to be 9.1 months, while the median overall survival (OS) reached 14.1 months, both indicative of significant antitumor activity within this patient cohort. In terms of safety, Garsorasib
exhibited a generally favorable tolerability profile [42]. The most common treatment-related adverse events included diarrhea, nausea, and elevated liver enzymes, which were predominantly of grade 1 or 2
severity.The synthesis of Garsorasib, depicted in Scheme 10, initiates with Suzuki-Miyaura coupling of Gars-001 and cyclopropylboronic acid, affording Gars-002 [43]. Gars-003 undergoes nucleophilic acylation with acryloyl chloride to yield Gars-004. TFA-mediated Boc deprotection of Gars-004 affords Gars-005. In parallel, Gars-006 is sequentially acylated with oxalyl chloride and aminated with ammonia to form Gars-007. DCE-mediated acylation of Gars-007, followed by concentration and coupling with Gars-002 in MeCN, produces Gars-008.KHMDS-catalyzed intramolecular cyclization of Gars-008 generates Gars-009. DIEA-catalyzed intermediate generation enables nucleophilic coupling with Gars-005 to assemble Gars-010. Final Suzuki-Miyaura coupling of Gars-010 with 3-fluoro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline delivers Garsorasib.
[40] W. Luo, J. Zhu, W. Zhang, A. Yu, W. Zhou, K. Xu, Efficacy and toxicity of drugs
targeting KRAS(G12C) mutation in non-small cell lung cancer: a meta-analysis,
Expert Rev. Anticancer Ther. 23 (2023) 1295–1303.
[41] Z. Li, X. Dang, D. Huang, S. Jin, W. Li, J. Shi, X. Wang, Y. Zhang, Z. Song, J. Zhang,
W. Zhuang, X. Liu, L. Jiang, X. Meng, M. Zhao, J. Zhou, L. Zhang, P. Wang, H. Luo,
J. Yang, S. Cang, X. Wang, L. Zhang, S. Lu, Garsorasib in patients with KRAS
(G12C)-mutated non-small-cell lung cancer in China: an open-label, multicentre,
single-arm, phase 2 trial, Lancet Respir. Med. 12 (2024) 589–598.
[42] Z. Li, Z. Song, Y. Zhao, P. Wang, L. Jiang, Y. Gong, J. Zhou, H. Jian, X. Dong,
W. Zhuang, S. Cang, N. Yang, J. Fang, J. Shi, J. Lu, R. Ma, P. Wu, Y. Zhang,
M. Song, C.W. Xu, Z. Shi, L. Zhang, Y. Wang, X. Wang, Y. Zhang, S. Lu, D-1553
(garsorasib), a potent and selective inhibitor of KRAS(G12C) in patients with
NSCLC: phase 1 study results, J. Thorac. Oncol. 18 (2023) 940–951.
[43] X. Dai, Y. Wang, Y. Jiang, Y. Liu, Z. Shi, Z. Wang, L. Tao, Z. Han, H. Niu, J. Weng,
Heterocyclic Compounds, Preparation Methods and Uses Thereof in the Treatment
of Cancers, 2020 CN112585129A.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
- New drugs approved by the NMPA in 2024: Synthesis and clinical applicationsPublication Name: European Journal of Medicinal ChemistryPublication Date: 2025-07-05PMID: 40262297DOI: 10.1016/j.ejmech.2025.117643
- D‐1553: A novel <scp>KRASG12C</scp> inhibitor with potent and selective cellular and in vivo antitumor activityPublication Name: Cancer SciencePublication Date: 2023-05-09PMCID: PMC10323112PMID: 37158138DOI: 10.1111/cas.15829
Methods of treating a ras protein-related disease or disorder
Publication Number: US-2025049810-A1
- Heterocyclic compounds, preparation methods and uses thereofPublication Number: US-2022226328-A1Priority Date: 2019-12-18
- Heterocyclic compounds, preparation methods and uses thereofPublication Number: WO-2020233592-A1Priority Date: 2019-05-21
- Heterocyclic compounds, preparation and methods and uses thereofPublication Number: US-11091481-B2Priority Date: 2019-05-21Grant Date: 2021-08-17
- Heterocyclic compounds, preparation and methods and uses thereofPublication Number: US-2021198255-A1Priority Date: 2019-05-21
- Heterocyclic compounds, preparation methods and uses thereofPublication Number: US-2021355125-A1Priority Date: 2019-05-21
- Medicament for treatment and/or prevention of cancerPublication Number: WO-2023008462-A1Priority Date: 2021-07-27
- Heterocyclic compounds, preparation methods and uses thereofPublication Number: US-2021260067-A1Priority Date: 2019-12-18
- Heterocyclic compounds, preparation methods and uses thereofPublication Number: WO-2021120045-A1Priority Date: 2019-12-18
- Heterocyclic compounds, preparation methods and uses thereofPublication Number: WO-2021121330-A1Priority Date: 2019-12-18
- Heterocyclic compounds, preparation methods and uses thereofPublication Number: US-11241437-B2Priority Date: 2019-12-18Grant Date: 2022-02-08
- Medicament for treatment and/or prevention of cancerPublication Number: AU-2022320304-A1Priority Date: 2021-07-27
- Medicament for treatment and/or prevention of cancerPublication Number: CA-3227706-A1Priority Date: 2021-07-27
- Medicines for the treatment and/or prevention of cancerPublication Number: CN-117677398-APriority Date: 2021-07-27
- Medicament for treatment and/or prevention of cancerPublication Number: EP-4378478-A1Priority Date: 2021-07-27
- Medicines for the treatment and/or prevention of cancerPublication Number: KR-20240041917-APriority Date: 2021-07-27
- Composition for preventing or treating c-myc-associated diseases through inhibition of expression of ubiquitin-specific protease 47 (usp47), and use thereofPublication Number: WO-2024085603-A1Priority Date: 2022-10-18
- Chimeric small molecules for labeling proteins with immunogenic moieties and methods of use thereofPublication Number: WO-2024081675-A2Priority Date: 2022-10-10
- Macrocyclic heterocycles and uses thereofPublication Number: US-11912708-B2Priority Date: 2022-04-20Grant Date: 2024-02-27
- Macrocyclic heterocycles and uses thereofPublication Number: US-2023339952-A1Priority Date: 2022-04-20
- Macrocyclic heterocycles and uses thereofPublication Number: WO-2023205701-A1Priority Date: 2022-04-20
- Composition for cancer prevention or treatment containing met inhibitor and k-ras inhibitorPublication Number: WO-2024191132-A1Priority Date: 2023-03-10
- Combination therapy and related methodsPublication Number: TW-202436340-APriority Date: 2023-02-06
- Combination therapy and related methodsPublication Number: US-2024343813-A1Priority Date: 2023-02-06
- Combination therapy and related methodsPublication Number: WO-2024167880-A1Priority Date: 2023-02-06
- A composition for preventing or treating c-Myc-related diseases through suppression of expression of USP47, and use thereofPublication Number: KR-20240055632-APriority Date: 2022-10-18
- Ectonucleotide pyrophosphatase/phosphodiesterase 1 (enpp1) inhibitor combinations and uses thereofPublication Number: WO-2025007955-A1Priority Date: 2023-07-06
- Methionine adenosyltransferase 2a (mat2a) inhibitor combinations and uses thereofPublication Number: WO-2024217502-A1Priority Date: 2023-04-19
- Combinations of kras inhibitor and atr inhibitor for the treatment of cancerPublication Number: WO-2024213699-A1Priority Date: 2023-04-14
- Anti-lipocalin-2 antibodies and uses thereofPublication Number: WO-2024191972-A2Priority Date: 2023-03-13
- Composition for Cancer Prevention or Treatment Including MET inhibitors and K-RAS inhibitorsPublication Number: KR-20240138493-APriority Date: 2023-03-10
- A kind of preparation method of 2,5,6-trichloronicotinic acidPublication Number: CN-117263854-APriority Date: 2023-09-15
- Protein tyrosine phosphatase degraders and uses thereofPublication Number: WO-2025030006-A2Priority Date: 2023-08-02
- Protein tyrosine phosphatase inhibitor combinationsPublication Number: WO-2025030008-A1Priority Date: 2023-08-02
- Methods of treating a ras related disease or disorderPublication Number: US-2025041295-A1Priority Date: 2023-07-14
- Methods of treating a ras related disease or disorderPublication Number: WO-2025019318-A2Priority Date: 2023-07-14
///////Garsorasib, D 1553, Chia Tai Tianqing, CHINA 2024, APPROVALS 2024, Anfangning, 2559761-14-5, P491NE9G6Z














