 MITAPIVAT
MITAPIVAT
CAS 1260075-17-9
MF C24H26N4O3S MW 450.55
UPDATE…. FDA APPROVE 2/17/2022 To treat hemolytic anemia in pyruvate kinase deficiency, Pyrukynd
8-Quinolinesulfonamide, N-[4-[[4-(cyclopropylmethyl)-1-piperazinyl]carbonyl]phenyl]-
N-[4-[[4-(Cyclopropylmethyl)-1-piperazinyl]carbonyl]phenyl]-8-quinolinesulfonamide
- Originator Agios Pharmaceuticals
- Class Antianaemics; Piperazines; Quinolines; Small molecules; Sulfonamides
- Mechanism of Action Pyruvate kinase stimulants
- Orphan Drug Status Yes – Inborn error metabolic disorders
- New Molecular Entity Yes
- Phase III Inborn error metabolic disorders
- Phase II Thalassaemia
- 27 Feb 2019 Agios Pharmaceuticals plans a phase III trial for Inborn error metabolic disorders (Pyruvate kinase deficiency) (Treatment-experienced) in the US, Brazil, Canada, Czech Republic, Denmark, France, Germany, Ireland, Italy, Japan, South Korea, Netherlands, Portugal, Spain, Switzerland, Thailand, Turkey and United Kingdom in March 2019 (NCT03853798) (EudraCT2018-003459-39)
- 11 Dec 2018 Phase-II clinical trials in Thalassaemia in Canada (PO) (NCT03692052)
- 29 Aug 2018 Chemical structure information added
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Mitapivat sulfate | N4JTA67V3O | Not Available | Not applicable |
Activator of pyruvate kinase isoenzyme M2 (PKM2), an enzyme involved in glycolysis. Since all tumor cells exclusively express the embryonic M2 isoform of PK, it is hypothesized that PKM2 is a potential target for cancer therapy. Modulation of PKM2 might also be effective in the treatment of obesity, diabetes, autoimmune conditions, and antiproliferation-dependent diseases.
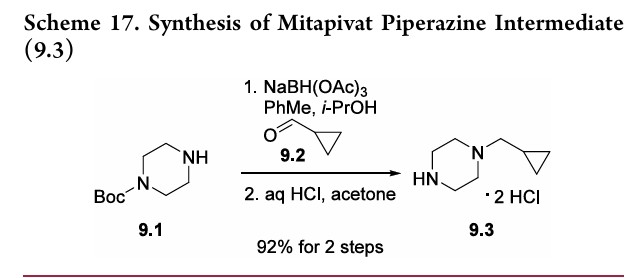
Agios Pharmaceuticals is developing AG-348 (in phase 3 , in June 2019), an oral small-molecule allosteric activator of the red blood cell-specific form of pyruvate kinase (PK-R), for treating PK deficiency and non-transfusion-dependent thalassemia. Mitapivat is a novel, first-in-class pyruvate kinase activator. It works to increase the activity of erythrocyte pyruvate kinase, a key enzyme involved in the survival of red blood cells. Defects in the pyruvate kinase enzyme in various red blood cells disorders lead to the lack of energy production for red blood cells, leading to lifelong premature destruction of red blood cells or chronic hemolytic anemia.1 On February 17, 2022, the FDA approved mitapivat as the first disease-modifying treatment for hemolytic anemia in adults with pyruvate kinase (PK) deficiency, a rare, inherited disorder leading to lifelong hemolytic anemia.6 Mitapivat has also been investigated in other hereditary red blood cell disorders associated with hemolytic anemia, such as sickle cell disease and alpha- and beta-thalassemia.1 SYN WO 20100331307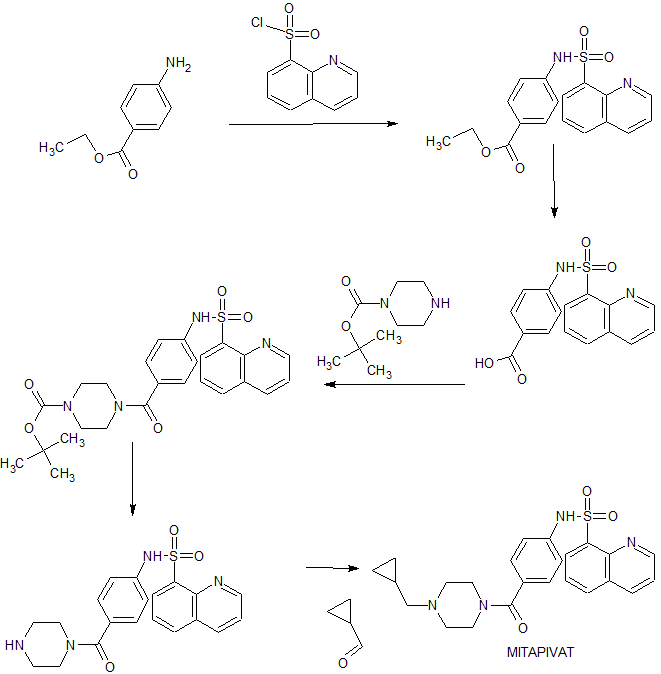
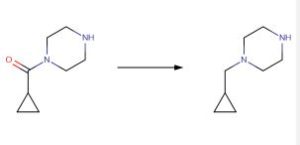 CAS 59878-57-8 TO CAS 57184-25-5
Eisai Co., Ltd., EP1508570, Lithium aluminium hydride (770 mg, 20.3 mmol) was suspended in tetrahydrofuran (150 mL), 1-(cyclopropylcarbonyl)piperazine (1.56 g, 10.1 mmol) was gradually added thereto, and the reaction mixture was heated under reflux for 30 minutes. The reaction mixture was cooled to room temperature, and 0.8 mL of water, 0.8 mL of a 15percent aqueous solution of sodium hydroxide and 2.3 mL of water were seque ntially gradually added thereto. The precipitated insoluble matter was removed by filtration through Celite, and the filtrate was evaporated to give the title compound (1.40g) as a colorless oil. The product was used for the synthesis of (8E,12E,14E)-7-((4-cyclopropylmethylpiperazin-1-yl)carbonyl)oxy-3,6,16,21-tetrahydroxy-6,10,12,16,20-pentamethyl-18,19-epoxytricosa-8,12,14-trien-11-olide (the co mpound of Example 27) without further purification.1H-NMR Spectrum (CDCl3,400MHz) delta(ppm): 0.09-0.15(2H,m), 0.48-0.56(2H,m),0.82-0.93(1H,m),2.25(2H,d,J=7.2Hz) 2.48-2.65(4H,m),2.90-2.99(4H,m).
CAS 59878-57-8 TO CAS 57184-25-5
Eisai Co., Ltd., EP1508570, Lithium aluminium hydride (770 mg, 20.3 mmol) was suspended in tetrahydrofuran (150 mL), 1-(cyclopropylcarbonyl)piperazine (1.56 g, 10.1 mmol) was gradually added thereto, and the reaction mixture was heated under reflux for 30 minutes. The reaction mixture was cooled to room temperature, and 0.8 mL of water, 0.8 mL of a 15percent aqueous solution of sodium hydroxide and 2.3 mL of water were seque ntially gradually added thereto. The precipitated insoluble matter was removed by filtration through Celite, and the filtrate was evaporated to give the title compound (1.40g) as a colorless oil. The product was used for the synthesis of (8E,12E,14E)-7-((4-cyclopropylmethylpiperazin-1-yl)carbonyl)oxy-3,6,16,21-tetrahydroxy-6,10,12,16,20-pentamethyl-18,19-epoxytricosa-8,12,14-trien-11-olide (the co mpound of Example 27) without further purification.1H-NMR Spectrum (CDCl3,400MHz) delta(ppm): 0.09-0.15(2H,m), 0.48-0.56(2H,m),0.82-0.93(1H,m),2.25(2H,d,J=7.2Hz) 2.48-2.65(4H,m),2.90-2.99(4H,m).
 CAS 91-22-5 TO CAD 18704-37-5
CAS 91-22-5 TO CAD 18704-37-5
Development Overview
Introduction
Mitapivat (designated AG 348), an orally available, first-in-class, small molecule stimulator of pyruvate kinase (PK), is being developed by Agios Pharmaceuticals for the treatment of pyruvate kinase deficiency (Inborn error metabolic disorders in development table) and thalassemia. Mitapivat is designed to activate the wild-type (normal) and mutated PK-R (the isoform of pyruvate kinase that is present in erythrocytes), in order to correct the defects in red cell glycolysis found within mutant cells. Clinical development is underway for inborn error metabolic disorders in the US, Spain and Denmark and for Thalassaemia in Canada.
Mitapivat emerged from Agios’ research programme focussed on the discovery of small molecule therapeutics for inborn metabolic disorders [see Adis Insight Drug Profile 800036791].
Key Development Milestones
In June 2018, Agios Pharmaceuticals initiated the phase III ACTIVATE trial to evaluate the efficacy and safety of orally administered mitapivat as compared with placebo in participants with pyruvate kinase deficiency (PKD), who are not regularly receiving blood transfusions (NCT03548220; AG348-C-006). The randomised, double-blind, placebo-controlled global trial intends to enrol 80 patients in the US, Canada, Denmark, France, Germany, Italy, Japan, South Korea, Netherlands, Poland, Portugal, Spain, Switzerland, Thailand and United Kingdom. The study design has two parts. Part 1 is a dose optimisation period where patients start at 5mg of mitapivat or placebo twice daily, with the flexibility to titrate up to 20mg or 50mg twice daily over a three month period to establish their individual optimal dose, as measured by maximum increase in hemoglobin levels. After the dose optimisation period, patients will receive their optimal dose for an additional three months in part 2. The primary endpoint of the study is the proportion of patients who achieve at least a 1.5 g/dL increase in haemoglobin sustained over multiple visits in part 2 of the trial
In February 2018, Agios Pharmaceuticals initiated the phase III ACTIVATE-T trial to assess the efficacy and safety of mitapivat in regularly transfused adult subjects with pyruvate kinase deficiency (Inborn error metabolism disorders in development table) (EudraCT2017-003803-22; AG348-C-007). The open label trial will enrol approximately 20 patients in Denmark and Spain and will expand to Canada, France, Italy, Japan, the Netherlands, the UK and the US
In December 2018, Agios Pharmaceuticals initiated a phase II study to assess the safety, efficacy, pharmacokinetics and pharmacodynamics of mitapivat (50mg and 100mg) for the treatment of patients with non-transfusion-dependent thalassemia (AG348-C-010; EudraCT2018-002217-35; NCT03692052). This study will include a 24-week core period followed by a 2-year extension period for eligible participants. The open-label trial intends to enrol approximately 17 patients. Enrolment has been initiated in Canada and may expand to the US and the UK
Agios Pharmaceuticals, in June 2015 initiated the phase II DRIVE PK trial to evaluate the safety, efficacy, pharmacokinetics and pharmacodynamics of mitapivat in adult transfusion-independent patients with pyruvate kinase deficiency (Inborn error metabolism disorders in development table) (AG348-C-003; NCT02476916). The trial will include two arms with 25 patients each. The patients in the first arm will receive 50mg twice daily, and the patients in the second arm will receive 300mg twice daily. The study will include a six-month dosing period with the opportunity for continued treatment beyond six months based on safety and clinical activity. The open-label, randomised trial completed enrolment of targeted 52 patients in the US, in November 2016. Preliminary data from the trial was presented at the 21st Congress of the European Haematology Association (EHA-2016). Updated results were presented by Agios at the 58th Annual Meeting and Exposition of the American Society of Haematology in December 2016. Based on results of the DRIVE PK trial, Agios plans to develop a registration path for mitapivat. Updated data from the trial was presented at the 22nd Congress of the European Haematology Association (EHA-2017)
In June 2018, Agios Pharmaceuticals completed a phase I trial in healthy male volunteers to assess the absorption, distribution, metabolism, excretion and absolute bioavailability of AG 348 (AG348-C-009; NCT03703505). Radiolabelled analytes of AG 348 ([14C]AG 348 and [13C6]AG 348) were administered in a single oral and intravenous dose on day 1. The open label trial was initiated in May 2018 and enrolled 8 volunteers in the US
In November 2017, Agios Pharmaceuticals completed a phase I trial that evaluated the relative bioavailability and safety of the mitapivat tablet and capsule formulations after single-dose administration in healthy adults (AG348-C-005; NCT03397329). The open-label trial enrolled 26 subjects in the US and was initiated in October 2017
In October 2017, Agios Pharmaceuticals completed a phase I trial that evaluated the pharmacokinetics, safety and effect on QTc interval of mitapivat in healthy volunteers (AG348-C-004; NCT03250598). This single-dose, open-label trial was initiated in August 2017 and enrolled 60 volunteers in the US
In November 2014, Agios completed a randomised, double-blind, placebo-controlled phase I trial that assessed the safety, pharmacokinetics and pharmacodynamics of multiple escalating doses of mitapivat in healthy volunteers (MAD; AG-348MAD; AG348-C-002; NCT02149966). Mitapivat was dosed daily for 14 days. The trial recruited 48 subjects in the US. In June 2015, positive results from the trial were presented at the 20th congress of the European Haematology Association (EHA-2015). Mitapivat showed a favourable pharmacokinetic profile with rapid absorption, low to moderate variability and a dose-proportional increase in exposure following multiple doses and serum hormone changes consistent with reversible aromatase inhibition were also observed
Agios Pharmaceuticals completed a randomised, double-blind, placebo-controlled phase I clinical trial of mitapivat in August 2014 (AG-348 SAD; AG348-C-001; NCT02108106). The study evaluated the safety, pharmacokinetics and pharmacodynamics of single escalating doses of the agent in healthy volunteers. Potential metabolic biomarkers were also explored. The trial enrolled 48 participants in the US
Patent Information
As of January 2018, Agios Pharmaceuticals owned approximately six issued US patents, 65 issued foreign patents, five pending US patent applications and 55 pending foreign patent applications in a number of jurisdictions directed to PK deficiency programme, including mitapivat (AG 348). The patents are valid till at least 2030
- Route of administrationPO
- FormulationTablet, unspecified
- ClassAntianaemics, Piperazines, Quinolines, Small molecules, Sulfonamides
- Mechanism of ActionPyruvate kinase stimulants
- WHO ATC codeA16A-X (Various alimentary tract and metabolism products)B03 (Antianemic Preparations)B06A (Other Hematological Agents)
- EPhMRA codeA16A (Other Alimentary Tract and Metabolism Products)B3 (Anti-Anaemic Preparations)B6 (All Other Haematological Agents)
- Chemical nameN-[4-[4-(cyclopropylmethyl)piperazine-1-carbonyl]phenyl]quinoline-8-sulfonamide
- Molecular formulaC24 H26 N4 O3 S
References
-
Agios Reports First Quarter 2017 Financial Results.
Media Release -
Agios Announces Initiation of Global Phase 3 Trial (ACTIVATE) of AG-348 in Adults with Pyruvate Kinase Deficiency Who Are Not Regularly Transfused.
Media Release -
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of AG-348 in Not Regularly Transfused Adult Subjects With Pyruvate Kinase Deficiency
ctiprofile -
Agios Provides Business Update on Discovery Research Strategy and Pipeline, Progress on Clinical Programs, Commercial Launch Preparations and Reports First Quarter 2018 Financial Results at Investor Day.
Media Release -
An Open-Label Study To Evaluate the Efficacy and Safety of AG-348 in Regularly Transfused Adult Subjects With Pyruvate Kinase (PK) Deficiency
ctiprofile -
A Phase 2, Open-label, Multicenter Study to Determine the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of AG-348 in Adult Subjects With Non-transfusion-dependent Thalassemia
ctiprofile -
Agios Announces Key Upcoming Milestones to Support Evolution to a Commercial Stage Biopharmaceutical Company in 2017.
Media Release -
Agios to Present Clinical and Preclinical Data at the 20th Congress of the European Hematology Association.
Media Release -
Agios Announces Updated Data from Fully Enrolled DRIVE PK Study Demonstrating AG-348s Potential as the First Disease-modifying Treatment for Patients with Pyruvate Kinase Deficiency.
Media Release -
Agios Announces New Data from AG-348 and AG-519 Demonstrating Potential for First Disease-modifying Treatment for Patients with PK Deficiency.
Media Release -
Agios Provides Update on PKR Program.
Media Release -
AG-348 Achieves Proof-of-Concept in Ongoing Phase 2 DRIVE-PK Study and Demonstrates Rapid and Sustained Hemoglobin Increases in Adults with Pyruvate Kinase Deficiency.
Media Release -
Agios Reports New, Final Data from Phase 1 Multiple Ascending Dose (MAD) Study in Healthy Volunteers for AG-348, an Investigational Medicine for Pyruvate Kinase (PK) Deficiency.
Media Release -
Grace RF, Layton DM, Galacteros F, Rose C, Barcellini W, Morton DH, et al. Results Update from the DRIVE PK Study: Effects of AG-348, a Pyruvate Kinase Activator, in Patients with Pyruvate Kinase Deficiency. ASH-Hem-2017 2017; abstr. 2194.
Available from: URL: https://ash.confex.com/ash/2017/webprogram/Paper102236.html -
A Phase 2, Open Label, Randomized, Dose Ranging, Safety, Efficacy, Pharmacokinetic and Pharmacodynamic Study of AG-348 in Adult Patients With Pyruvate Kinase Deficiency
ctiprofile -
A Phase I, Open-label Study to Evaluate the Absorption, Distribution, Metabolism, and Excretion and to Assess the Absolute Bioavailability of AG-348 in Healthy Male Subjects Following Administration of a Single Oral Dose of [14C]AG-348 and Concomitant Single Intravenous Microdose of [13C6]AG-348
ctiprofile -
A Phase 1, Randomized, Open-Label, Two-Period Crossover Study Evaluating the Relative Bioavailability and Safety of the AG-348 Tablet and Capsule Formulations After Single-Dose Administration in Healthy Adults
ctiprofile -
A Phase 1, Single-Dose, Open-Label Study to Characterize and Compare the Pharmacokinetics, Safety, and Effect on QTc Interval of AG-348 in Healthy Subjects of Japanese Origin and Healthy Subjects of Non-Asian Origin
ctiprofile -
Agios Pharmaceuticals Initiates Multiple Ascending Dose Trial in Healthy Volunteers of AG-348 for the Potential Treatment of PK Deficiency, a Rare, Hemolytic Anemia.
Media Release -
A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose, Safety, Pharmacokinetic, and Pharmacodynamic Study of Orally Administered AG-348 in Healthy Volunteers
ctiprofile -
Agios Initiates Phase 1 Study of AG-348, a First-in-class PKR Activator, for Pyruvate Kinase Deficiency.
Media Release -
A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Safety, Pharmacokinetic and Pharmacodynamic Study of Orally Administered AG-348 in Healthy Volunteers
ctiprofile -
Agios Pharmaceuticals Reports First Quarter 2014 Financial Results.
Media Release -
Agios Pharmaceuticals Reports Third Quarter 2013 Financial Results.
Media Release -
Agios Pharmaceuticals to Present Preclinical Research at the 2013 American Society of Hematology Annual Meeting.
Media Release -
Agios Presents Preclinical Data from Lead Programs at American Society of Hematology Annual Meeting.
Media Release -
Agios Pharmaceuticals Form 10-K, February 2018. Internet-Doc 2018;.
Available from: URL: https://www.sec.gov/Archives/edgar/data/1439222/000143922218000004/agio-123117x10k.htm -
Agios Outlines Key 2018 Priorities Expanding Clinical and Research Programs to Drive Long Term Value.
Media Release -
Grace RF, Layton DM, Galacteros F, Rose C, Barcellini W, Morton DH, et al. Effects of Ag-348, a Pyruvate Kinase Activator, in Patients with Pyruvate Kinase Deficiency: Updated Results from the Drive Pk Study. EHA-2017 2017; abstr. S451.
Available from: URL: https://learningcenter.ehaweb.org/eha/2017/22nd/181738/rachael.f.grace.effects.of.ag-348.a.pyruvate.kinase.activator.in.patients.with.html?f=m3e1181l15534 -
Agios Presents Updated Data from DRIVE PK Study Demonstrating AG-348 is Well-Tolerated and Results in Clinically Relevant, Rapid and Sustained Hemoglobin Increases in Patients with Pyruvate Kinase Deficiency.
Media Release
Medical uses
Mitapivat is indicated for the treatment of hemolytic anemia in adults with pyruvate kinase deficiency.[1][3]Pharmacology
Mechanism of action
Mitapivat binds to and activates pyruvate kinase, thereby enhancing glycolytic pathway activity, improving adenosine triphosphate (ATP) levels and reducing 2,3-diphosphoglycerate (2,3-DPG) levels.[4] Mutations in pyruvate kinase cause deficiency in pyruvate kinase which prevents adequate red blood cell (RBC) glycolysis, leading to a buildup of the upstream glycolytic intermediate 2,3-DPG and deficiency in the pyruvate kinase product ATP.[4][5]Society and culture
Names
Mitapivat is the international nonproprietary name (INN).[6]References
- ^ Jump up to:a b c d e f https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216196s000lbl.pdf
- ^ “Agios Announces FDA Approval of Pyrukynd (mitapivat) as First Disease-Modifying Therapy for Hemolytic Anemia in Adults with Pyruvate Kinase Deficiency” (Press release). Agios Pharmaceuticals. 17 February 2022. Retrieved 19 February 2022 – via GlobeNewswire.
- ^ Jump up to:a b https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/216196Orig1s000ltr.pdf
- ^ Jump up to:a b “Mitapivat (Code C157039)”. NCI Thesaurus. 31 January 2022. Retrieved 19 February 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ “PK-R allosteric activator AG-348”. NCI Drug Dictionary. National Cancer Institute. Retrieved 19 February 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ World Health Organization (2017). “International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78”. WHO Drug Information. 31 (3): 539. hdl:10665/330961.
Further reading
- Kung C, Hixon J, Kosinski PA, Cianchetta G, Histen G, Chen Y, et al. (September 2017). “AG-348 enhances pyruvate kinase activity in red blood cells from patients with pyruvate kinase deficiency”. Blood. 130 (11): 1347–1356. doi:10.1182/blood-2016-11-753525. PMC 5609468. PMID 28760888.
- Rab MA, Van Oirschot BA, Kosinski PA, Hixon J, Johnson K, Chubukov V, et al. (January 2021). “AG-348 (Mitapivat), an allosteric activator of red blood cell pyruvate kinase, increases enzymatic activity, protein stability, and ATP levels over a broad range of PKLR genotypes”. Haematologica. 106 (1): 238–249. doi:10.3324/haematol.2019.238865. PMC 7776327. PMID 31974203.
External links
- “Mitapivat sulfate”. Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03548220 for “A Study to Evaluate Efficacy and Safety of AG-348 in Not Regularly Transfused Adult Participants With Pyruvate Kinase Deficiency (PKD)” at ClinicalTrials.gov
- Clinical trial number NCT03559699 for “A Study Evaluating the Efficacy and Safety of AG-348 in Regularly Transfused Adult Participants With Pyruvate Kinase Deficiency (PKD)” at ClinicalTrials.gov
| Clinical data | |
|---|---|
| Trade names | Pyrukynd |
| Other names | AG-348, Mitapivat sulfate (USAN US) |
| License data | |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| Chemical and physical data | |
| Formula | C24H26N4O3S |
| Molar mass | 450.56 g·mol−1 |
| 3D model (JSmol) | |














