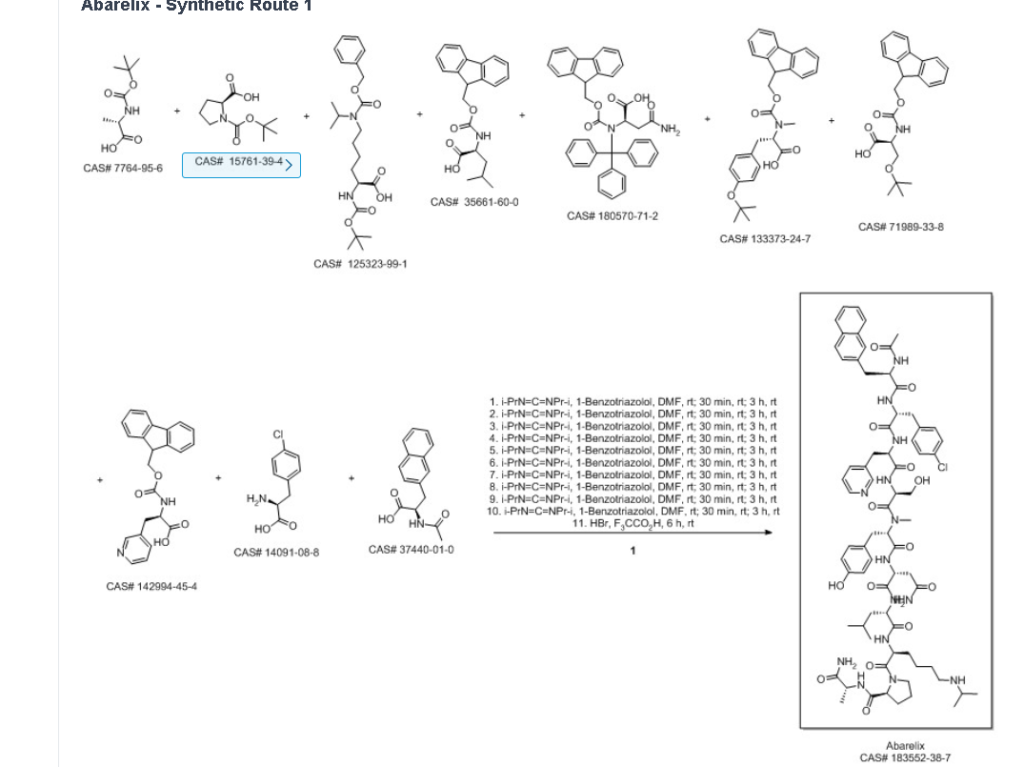
Abarelix
CAS 183552-38-7
785804-17-3 (acetate) 183552-38-7 (free base)
PPI149, PPI-149, PPI 149, R3827, R-3827, R 3827, Abarelix, Abarelix acetate, Plenaxis,
W486SJ5824
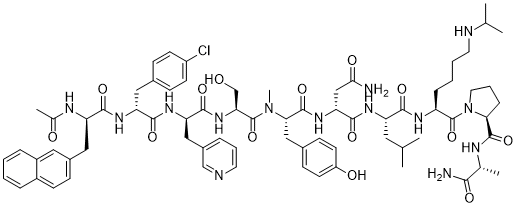
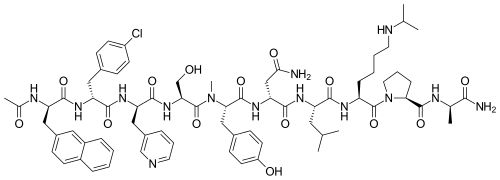
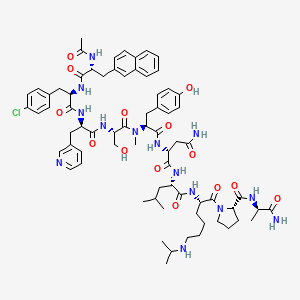
Chemical Formula: C72H95ClN14O14
Exact Mass: 1414.6841
Molecular Weight: 1416.06
Ac-D-Nal-[D-(pCl)Phe]-D-Pal-Ser-[Nalpha-Me-Tyr]-D-Asn-Leu-ILys-Pro-DAla-NH2

(2R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-[[(2R)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]-methylamino]-3-(4-hydroxyphenyl)propanoyl]amino]-N-[(2S)-1-[[(2S)-1-[(2S)-2-[[(2R)-1-amino-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-1-oxo-6-(propan-2-ylamino)hexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]butanediamide
(2R)-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-[[(2R)-2-acetamido-3-naphthalen-2-ylpropanoyl]amino]-3-(4-chlorophenyl)propanoyl]amino]-3-pyridin-3-ylpropanoyl]amino]-3-hydroxypropanoyl]-methylamino]-3-(4-hydroxyphenyl)propanoyl]amino]-N-[(2S)-1-[[(2S)-1-[(2S)-2-[[(2R)-1-amino-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-1-oxo-6-(propan-2-ylamino)hexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]butanediamide
Abarelix is a synthetic decapeptide and antagonist of naturally occurring gonadotropin-releasing hormone (GnRH). Abarelix directly and competitively binds to and blocks the gonadotropin releasing hormone receptor in the anterior pituitary gland, thereby inhibiting the secretion and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH). In males, the inhibition of LH secretion prevents the release of testosterone. As a result, this may relieve symptoms associated with prostate hypertrophy or prostate cancer, since testosterone is required to sustain prostate growth.
Abarelix, sold under the brand name Plenaxis, is an injectable gonadotropin-releasing hormone antagonist (GnRH antagonist) which is marketed in Germany and the Netherlands. It is primarily used in oncology to reduce the amount of testosterone made in patients with advanced symptomatic prostate cancer for which no other treatment options are available.[2][3]
It was originally marketed by Praecis Pharmaceuticals as Plenaxis,[2] and is now marketed by Speciality European Pharma in Germany[4] after receiving a marketing authorization in 2005. The drug was introduced in the United States in 2003, but was discontinued in this country in May 2005 due to poor sales and a higher-than-expected incidence of severe allergic reactions.[5] It remains marketed in Germany and the Netherlands however.[6]
Pat
https://patents.google.com/patent/CN107778354B/en
Example 1: synthesis of peptide resin 1
Dissolving 0.15mol of Fmoc-D-Ala and 0.15mol of HOBt by using a proper amount of DMF; and adding 0.15mol DIC slowly into the protected amino acid DMF solution under stirring, and reacting for 30 minutes under stirring at room temperature to obtain an activated protected amino acid solution for later use.
Taking 0.05mol of MOBHA resin (the substitution value is about 0.6mmol/g), swelling with DMF for 25 minutes, washing and filtering, adding the activated solution, stirring at room temperature for reaction for 3 hours, pumping out the reaction solution, washing with DMF for 3 times, washing with DCM for 3 times, wherein the washing time is 3min each time, obtaining Fmoc-D-Ala-MOBHA resin, namely the peptide resin 1, removing Fmoc protection with 20% PIP/DMF solution for 25 minutes before carrying out the next coupling reaction, washing and filtering to obtain the D-Ala-MOBHA resin.
Example 2: synthesis of peptide resin 1
Dissolving 0.15mol of Boc-D-Ala and 0.15mol of HOBt with a proper amount of DMF; and adding 0.15mol DIC slowly into the protected amino acid DMF solution under stirring, and reacting for 30 minutes under stirring at room temperature to obtain an activated protected amino acid solution for later use.
Taking 0.05mol of MOBHA resin (the substitution value is about 0.6mmol/g), swelling with DMF for 25 minutes, washing and filtering, adding an activated Fmoc-D-Ala solution, stirring at room temperature for 3 hours, pumping out the reaction solution, washing 3 times with DMF, washing 3 times with DCM, wherein each washing time is 3min, obtaining Boc-D-Ala-MOBHA resin, namely peptide resin 1, deprotecting with 30% TFA/DCM solution for 30 minutes, neutralizing with DIEA/DCM solution, washing and filtering with DMF and DCM, and obtaining D-Ala-MOBHA resin.
Example 3: synthesis of Abarelix peptide resin
Dissolving 0.15mol of Fmoc-Pro and 0.15mol of HOBt in a proper amount of DMF; and adding 0.15mol DIC slowly into the protected amino acid DMF solution under stirring, and reacting for 30 minutes under stirring at room temperature to obtain an activated protected amino acid solution for later use.
Adding the activated Fmoc-Pro solution into the peptide resin 1 obtained in example 1, stirring at room temperature for reaction for 3 hours, pumping out the reaction solution, washing with DMF for 3 times, washing with DCM for 3 minutes each time, removing Fmoc protection with 20% PIP/DMF solution for 25 minutes, washing and filtering to obtain Pro-D-Ala-MOBHA resin.
Boc-Lys (iPr, Z), Fmoc-Leu, Fmoc-D-Asn (Trt), Fmoc-N-Me-Tyr (tBu), Fmoc-Ser (tBu), Fmoc-D-Pal, Fmoc-D-Cpa and Ac-D-Nal are sequentially added in the same method, and the Abarelix peptide resin, Ac-D-Nal-D-Cpa-D-Pal-Ser (tBu) -N-Me-Tyr (tBu) -D-Asn (Trt) -Leu-Lys (iPr, Z) -Pro-D-Ala-MOBHA resin are obtained by washing and filtering.
Example 4: synthesis of Abarelix peptide resin
Dissolving 0.15mol of Boc-Pro and 0.15mol of HOBt by using a proper amount of DMF; and adding 0.15mol DIC slowly into the protected amino acid DMF solution under stirring, and reacting for 30 minutes under stirring at room temperature to obtain an activated protected amino acid solution for later use.
Adding the activated Boc-Pro solution into the peptide resin 1 obtained in example 1, stirring at room temperature for reaction for 3 hours, pumping out the reaction solution, washing with DMF for 3 times, washing with DCM for 3min each time, deprotecting with 30% TFA/DCM solution for 30 minutes, neutralizing with DIEA/DCM solution, washing with DMF and DCM, and filtering to obtain Pro-D-Ala-MBHA resin.
Boc-Lys (iPr, Z), Fmoc-Leu, Fmoc-D-Asn (Trt), Fmoc-N-Me-Tyr (tBu), Fmoc-Ser (tBu), Fmoc-D-Pal, Fmoc-D-Cpa and Ac-D-Nal are sequentially added in the same method, and the Abarelix peptide resin, Ac-D-Nal-D-Cpa-D-Pal-Ser (tBu) -N-Me-Tyr (tBu) -D-Asn (Trt) -Leu-Lys (iPr, Z) -Pro-D-Ala-MOBHA resin are obtained by washing and filtering.
Example 5: preparation of crude Abarelix
Taking the abarelix peptide resin prepared in the example 3, adding 8% HBr/TFA solution (acidolysis solution 10mL/g abarelix resin), stirring and reacting for 6 hours, filtering and collecting filtrate, washing the resin with a small amount of TFA for 3 times, combining the filtrates, concentrating under reduced pressure, adding anhydrous ether for precipitation, washing the precipitate with anhydrous ether for 3 times, and draining to obtain white-like powder, namely a crude product of abarelix, wherein the purity of the crude product is 79.3%.
Example 6: preparation of crude Abarelix
Taking the abarelix peptide resin prepared in the example 4, adding 8% HBr/TFA solution (acidolysis solution 10mL/g abarelix resin), stirring and reacting for 6 hours, filtering and collecting filtrate, washing the resin with a small amount of TFA for 3 times, combining the filtrates, concentrating under reduced pressure, adding anhydrous ether for precipitation, washing the precipitate with anhydrous ether for 3 times, and draining to obtain white-like powder, namely a crude product of abarelix, wherein the purity of the crude product is 77.4%.
Example 7: purification and trans-salt conversion of crude Abarelix
Taking the crude Abarelix product obtained in the example 5, dissolving the Abarelix product in 20 percent acetic acid solution, filtering the solution by using a 0.45 mu m microporous membrane, and purifying for later use;
purifying by high performance liquid chromatography, wherein a chromatographic filler is 10 mu m reverse phase C18, a mobile phase system is 0.1% TFA/water solution-0.1% TFA/acetonitrile solution, a chromatographic column with the flow rate of 77mm x 250mm is 90mL/min, eluting by a gradient system, circularly sampling and purifying, sampling a crude product solution in the chromatographic column, starting the mobile phase for elution, collecting a main peak, and evaporating acetonitrile to obtain an abarelix purified intermediate concentrated solution;
taking the Abarelix purified intermediate concentrated solution, and filtering with a 0.45-micrometer filter membrane for later use;
performing salt exchange by high performance liquid chromatography, wherein the mobile phase system is 1% acetic acid/water solution-acetonitrile, the purification is performed by reversed phase C18 with chromatographic packing of 10 μm, the flow rate of a chromatographic column of 77mm × 250mm is 90mL/min, gradient elution and circular sample loading method are adopted, the sample is loaded in the chromatographic column, the mobile phase elution is started, the chromatogram is collected, the change of the absorbance is observed, the main peak of salt exchange is collected and the purity is detected by analyzing the liquid phase, the main peak solutions of salt exchange are combined, the concentration is performed under reduced pressure to obtain the aqueous solution of abarelix acetic acid, and freeze drying is performed to obtain 39.4g abarelix pure product
The total yield was 55.6%, molecular weight: 1417.2, purity: 99.6%, maximum single impurity of 0.13%, no toxic hydantoin degradation products were detected.
Example 8: purification and trans-salt conversion of crude Abarelix
Taking the crude Abarelix product obtained in the example 6, dissolving the Abarelix product by using a purification mobile phase A, and filtering the solution by using a 0.45 mu m microporous filter membrane to purify the Abarelix product for later use;
purifying by high performance liquid chromatography, wherein a chromatographic filler is 10 mu m reverse phase C18, a mobile phase system is 0.1% TFA/water solution-0.1% TFA/acetonitrile solution, a chromatographic column with the flow rate of 77mm x 250mm is 90mL/min, eluting by a gradient system, circularly sampling and purifying, sampling a crude product solution in the chromatographic column, starting the mobile phase for elution, collecting a main peak, and evaporating acetonitrile to obtain an abarelix purified intermediate concentrated solution;
taking the Abarelix purified intermediate concentrated solution, and filtering with a 0.45-micrometer filter membrane for later use;
performing salt exchange by adopting a high performance liquid chromatography, wherein a mobile phase system is 1% acetic acid/water solution-acetonitrile, a chromatographic filler for purification is reversed phase C18 with the diameter of 10 mu m, the flow rate of a chromatographic column with the diameter of 77mm × 250mm is 90mL/min, a gradient elution method and a circular sample loading method are adopted, loading the chromatographic column, starting the mobile phase elution, collecting a spectrum, observing the change of the absorbance, collecting a main salt exchange peak, detecting the purity by using an analysis liquid phase, combining main salt exchange peak solutions, concentrating under reduced pressure to obtain an abarelix acetic acid water solution, and performing freeze drying to obtain 41.7g of an abarelix pure product.
The total yield is 58.9%, molecular weight: 1417.0, purity: 99.5%, maximum single impurity 0.09%, no toxic hydantoin degradation products were detected.
SYN
Ma, Zhonggang; Guo, Dewen; Zeng, Dezhi; Wen, Yongjun. Method for synthesizing abarelix. Assignee Chengdu Shengnuo Biopharm Co., Ltd.. 2018.



AS ON JUNE2025 4.45 LAKHS VIEWS ON BLOG WORLDREACH AVAILABLEFOR YOUR ADVERTISEMENT

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@gmail.com

……
1: Tombal B. New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. BJU Int. 2012 Mar;109(6):E16; author reply E16-7. doi: 10.1111/j.1464-410X.2012.10983.x. PubMed PMID: 22360806.
2: Garnick MB, Mottet N. New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. BJU Int. 2012 Aug;110(4):499-504. doi: 10.1111/j.1464-410X.2011.10708.x. Epub 2011 Nov 16. PubMed PMID: 22093775.
3: Koechling W, Hjortkjaer R, Tankó LB. Degarelix, a novel GnRH antagonist, causes minimal histamine release compared with cetrorelix, abarelix and ganirelix in an ex vivo model of human skin samples. Br J Clin Pharmacol. 2010 Oct;70(4):580-7. doi: 10.1111/j.1365-2125.2010.03730.x. PubMed PMID: 20840449; PubMed Central PMCID: PMC2950992.
4: Retraction statement: Reconstitution of Plenaxis® (Abarelix) 100 mg for injection is more effective with a vortex-like mixer than when performed manually. J Pharm Pract. 2010 Feb;23(1):78. doi: 10.1177/0897190009360369. PubMed PMID: 21507797.
5: Kirby RS, Fitzpatrick JM, Clarke N. Abarelix and other gonadotrophin-releasing hormone antagonists in prostate cancer. BJU Int. 2009 Dec;104(11):1580-4. doi: 10.1111/j.1464-410X.2009.08924.x. Review. PubMed PMID: 20053189.
6: Debruyne F, Bhat G, Garnick MB. Abarelix for injectable suspension: first-in-class gonadotropin-releasing hormone antagonist for prostate cancer. Future Oncol. 2006 Dec;2(6):677-96. Review. PubMed PMID: 17155895.
7: Beer TM, Ryan C, Bhat G, Garnick M; Abarelix Study Group. Dose-escalated abarelix in androgen-independent prostate cancer: a phase I study. Anticancer Drugs. 2006 Oct;17(9):1075-9. PubMed PMID: 17001181.
8: Hogle WP. Abarelix (plenaxis). Clin J Oncol Nurs. 2004 Dec;8(6):663-5. PubMed PMID: 15637961.
9: Mongiat-Artus P, Teillac P. Abarelix: the first gonadotrophin-releasing hormone antagonist for the treatment of prostate cancer. Expert Opin Pharmacother. 2004 Oct;5(10):2171-9. Review. PubMed PMID: 15461552.
10: Wong SL, Lau DT, Baughman SA, Fotheringham N, Menchaca D, Garnick MB. Pharmacokinetics and pharmacodynamics of a novel depot formulation of abarelix, a gonadotropin-releasing hormone (GnRH) antagonist, in healthy men ages 50 to 75. J Clin Pharmacol. 2004 May;44(5):495-502. PubMed PMID: 15102870.
References
- “Abarelix”. PubChem. 2017-07-29.
- “Abarelix”. Drugs.com. Archived from the original on 2018-02-10. Retrieved 2018-01-23.
- Boccon-Gibod L, van der Meulen E, Persson BE (June 2011). “An update on the use of gonadotropin-releasing hormone antagonists in prostate cancer”. Therapeutic Advances in Urology. 3 (3): 127–40. doi:10.1177/1756287211414457. PMC 3159401. PMID 21904569.
- Pharmazeutische Zeitung online: Abarelix (in German)
- Minev B (13 January 2011). Cancer Management in Man: Chemotherapy, Biological Therapy, Hyperthermia and Supporting Measures. Springer Science & Business Media. pp. 182–. ISBN 978-90-481-9704-0.
- “Abarelix”. Drugs.com. Archived from the original on 2019-08-29. Retrieved 2018-08-27.
| Clinical data | |
|---|---|
| Trade names | Plenaxis |
| AHFS/Drugs.com | Monograph |
| Routes of administration | Intramuscular injection |
| Drug class | GnRH analogue; GnRH antagonist; Antigonadotropin |
| ATC code | L02BX01 (WHO) |
| Pharmacokinetic data | |
| Protein binding | 96–99% |
| Identifiers | |
| IUPAC name | |
| CAS Number | 183552-38-7 |
| PubChem CID | 16131215 |
| IUPHAR/BPS | 1188 |
| DrugBank | DB00106 |
| ChemSpider | 10482301 |
| UNII | W486SJ5824 |
| KEGG | D02738 |
| ChEBI | CHEBI:337298 |
| ChEMBL | ChEMBL1252 |
| CompTox Dashboard (EPA) | DTXSID20171443 |
| Chemical and physical data | |
| Formula | C72H95ClN14O14 |
| Molar mass | 1416.09 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| SMILES | |
| InChI | |
//////Abarelix, PPI149, PPI-149, PPI 149, R3827, R-3827, R 3827, Abarelix, Abarelix acetate, Plenaxis,
W486SJ5824
O=C(N[C@@H](CC(C)C)C(N[C@@H](CCCCNC(C)C)C(N1[C@H](C(N[C@H](C)C(N)=O)=O)CCC1)=O)=O)[C@H](NC([C@@H](N(C([C@@H](NC([C@H](NC([C@H](NC([C@H](NC(C)=O)CC2=CC=C3C=CC=CC3=C2)=O)CC4=CC=C(Cl)C=C4)=O)CC5=CC=CN=C5)=O)CO)=O)C)CC6=CC=C(O)C=C6)=O)CC(N)=O














