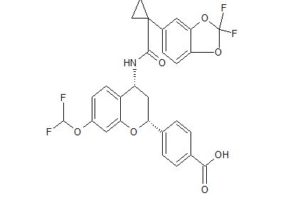
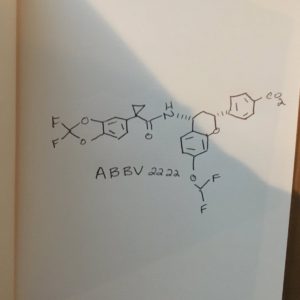
ABBV 2222
Benzoic acid, 4-[(2R,4R)-4-[[[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl]amino]-7-(difluoromethoxy)-3,4-dihydro-2H-1-benzopyran-2-yl]-
4-[(2R,4R)-4-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}- amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoic acid
CAS 1918143-53-9
| Philip R. Kym, Xueqing Wang, Xenia B. Searle, Bo Liu, Ming C. Yeung, Robert J. Altenbach, Eric Voight, Andrew Bogdan, John R. Koenig, Weniger « | |
| Abbvie Inc. |
DESCRIPTION
Cystic fibrosis (CF), one of the most common autosomal recessive genetic diseases in the Caucasian population, is caused by loss of function mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene, which is located on chromosome 7 (http://www.cff.org/AboutCF/; Rowe S. M et al. (2005); N Eng J Med. (352), 1992-2001). Approximately 1:3500 and 1:3000 infants born in the United States and in Europe, respectively, are affected by CF, resulting in ˜75,000 cases worldwide, ˜30,000 of which are in the United State. Approximately 1,000 new cases of CF are diagnosed each year, with more than 75% of patients being diagnosed by 2 years of age. Nearly half the CF population is currently 18 years of age and older. The CFTR protein (Gregory, R. J. et al. (1990) Nature 347:382-386; Rich, D. P. et al. (1990) Nature 347:358-362; Riordan, J. R. et al. (1989) Science 245:1066-1073) is a cAMP/ATP-mediated ion channel expressed in a variety of cell types, including secretory and absorptive epithelial cells. CFTR regulates chloride and bicarbonate anion flux across the cell membrane, maintaining electro neutrality and osmolarity across the epithelial membrane (Quinton, P. M. (1990), FASEB J. 4: 2709-2727). CFTR is also responsible for regulating the activity of other ion channels and proteins (Guggino, W. B. et al. (2006), Nat Revs Molecular Cell Biology 7, 426-436).
Aberrations in CFTR function result in imbalance of the airway surface liquid, leading to mucus dehydration, inflammation, recurrent bacterial infection and irreversible lung damage, which lead to premature death in affected patients. Besides respiratory disease, CF patients suffer from gastrointestinal problems and pancreatic insufficiency. The majority of males (95%) with cystic fibrosis are infertile as a result of azoospermia caused by altered vas deferens; which may be absent, atrophic, or fibrotic. Fertility is also decreased among females with cystic fibrosis due to abnormal cervical mucus.
The F508del mutation, the most common of the approximately 1900 identified polymorphisms in CFTR, results in defective processing of CFTR in the endoplasmic reticulum (ER) (http://www.cftr2.org/index.php). Approximately 90% of the CF patients carry at least one copy of the F508del mutation (deletion of a phenylalanine on position 508), and 50%-60% of the patients are homozygous for this mutation. The defective processing of CFTR results in early CFTR degradation, which leads to reduced trafficking or absence of the protein on the membrane. As there have been over 100 CF disease-causing mutations identified, they have been classified according to their phenotypic consequences and belong to synthesis, maturation, regulation, conductance, reduced number due to quantity and reduced number due to stability classifications.
Current CF drug discovery efforts focus upon developing two classes of compounds to modulate CFTR. One class, called Correctors, helps to overcome the folding defects of the mutated CFTR protein to promote its maturation resulting in higher cell surface expression. The other classes of compounds, called Potentiators, help overcome the defective regulation and/or conductance of the protein by increasing the probability of channel opening on the membrane surface.
In addition, as the modulation of CFTR protein mutations to promote proper protein folding is beneficial for CF, there are other diseases mediated by CFTR. For example, Sjögren’s Syndrome (SS), an autoimmune disorder that results in symptoms of xerostomia (dry mouth) and keratoconjunctivitis sicca (KCS, dry eyes) may result from dysregulation of moisture producing glands throughout the body. Chronic obstructive lung disease (COLD), or chronic obstructive airway disease (COAD), which is a progressive and irreversible airflow limitation in the airways is result of several physiologic abnormalities, including mucus hyper secretion and impaired mucociliary secretion. Increasing the anion secretion by CFTR potentiators have been suggested to overcome these phenotypic complexities with Sjögren’s Syndrome by increasing the corneal hydration and by overcoming the impaired mucociliary secretion in COAD (Bhowmik A, et al. (2009) Vol. 103(4), 496-502; Sloane P, et al. PLOS One (2012) Vol 7(6), 239809 (1-13)).
STEP 1
(R)-methyl 4-(7-hydroxy-4-oxochroman-2-yl)benzoate
RXN……….By reacting 7-hydroxy-4H-chromen-4-one AND (4-(methoxycarbonyl)phenyl)boronic acid
STEP 2
(R)-methyl 4-(7-hydroxy-4-(methoxyimino)chroman-2-yl)benzoate
Reacting ABOVE compd and O-methylhydroxylamine,
STEP 3
Methyl 4-((2R,4R)-4-amino-7-hydroxychroman-2-yl)benzoate
reacting ABOVE compd with 5% platinum (0.05 equivalent) on carbon in acetic acid. The reaction was stirred at room temperature under hydrogen
THEN STEP 4
Methyl 4-((2R,4R)-4-amino-7-hydroxychroman-2-yl)benzoate isolated AS trifluroroacetic acid salt
STEP 5
methyl 4-((2R,4R)-4-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanec- arboxamido)-7-hydroxychroman-2-yl)benzoate
by reacting 1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxylic acid and HATU (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate, the ABOVE compound AND N-ethyl-N-isopropylpropan-2-amine
STEP 6
Methyl 4-((2R,4R)-4-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanec- arboxamido)-7-(difluoromethoxy)chroman-2-yl)benzoate
by reacting ABOVE compound and diethyl(bromodifluoromethyl)phosphonate
AND FINAL STEP7 is ESTER HYDROLYSIS USING lithium hydroxide to get ABBV 2222

Example 122
4-[(2R,4R)-4-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}- amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoic acid
[1880] To Example 123E (130 mg, 0.227 mmol) in methanol (2 mL) and water (0.5 mL) was added lithium hydroxide (32.6 mg, 1.360 mmol). The mixture was stirred at 35.degree. C. for 4 hours, LC/MS showed the conversion was complete. Solvent was removed under reduced pressure and water (2 mL) was added. The pH of the mixture was adjusted to pH 1-2 with the addition of 2 M HCl. The precipitated white solid was collected by filtration, and dried to provide the title compound (110 mg, 0.197 mmol, 87% yield). .sup.1H NMR (400 MHz, CDCl.sub.3) .delta. 8.17-8.03 (m, 2H), 7.49 (d, J=8.2 Hz, 2H), 7.16-6.99 (m, 4H), 6.73-6.67 (m, 2H), 6.38 (d, J=73.6 Hz, 1H), 5.48 (td, J=10.4, 6.1 Hz, 1H), 5.36 (d, J=8.8 Hz, 1H), 5.31-5.21 (m, 1H), 2.52 (ddd, J=13.3, 6.0, 2.2 Hz, 1H), 1.86-1.71 (m, 2H), 1.68-1.60 (m, 1H), 1.10 (q, J=3.7, 2.4 Hz, 2H); MS (ESI-) m/z=558 (M-H).sup.-.
Example 123
methyl 4-[(2R,4R)-4-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]ca- rbonyl}amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoate
Example 123A
(R)-methyl 4-(7-hydroxy-4-oxochroman-2-yl)benzoate
[1881] A mixture of bis(2,2,2-trifluoroacetoxy)palladium (271 mg, 0.816 mmol), (S)-4-(tert-butyl)-2-(pyridin-2-yl)-4,5-dihydrooxazole (200 mg, 0.979 mmol), ammonium hexafluorophosphate(V) (798 mg, 4.90 mmol), (4-(methoxycarbonyl)phenyl)boronic acid (2203 mg, 12.24 mmol) and dichloroethane (8 mL) in a 20 mL vial was stirred for 5 minutes at room temperature, followed by the addition of 7-hydroxy-4H-chromen-4-one (CAS 59887-89-7, MFCD00209371, 1323 mg, 8.16 mmol) and water (256 mg, 14.19 mmol). The vial was capped and the mixture was stirred at 60.degree. C. overnight. The reaction gradually turned black, with Pd plated out on the sides of the vial. The mixture was filtered through a plug of celite and eluted with ethyl acetate to give a red solution which was washed with brine. The solvent was removed in vacuo and the crude material was chromatographed using a 100 g silica gel cartridge and eluted with a gradient of 5-40% ethyl acetate in heptane to provide the title compound (1.62 g, 66.6% yield). .sup.1H NMR (400 MHz, CDCl.sub.3) .delta. 8.15-8.04 (m, 2H), 7.87 (d, J=8.7 Hz, 1H), 7.60-7.49 (m, 2H), 6.62-6.45 (m, 2H), 5.87 (s, 1H), 5.53 (dd, J=12.8, 3.2 Hz, 1H), 3.94 (s, 3H), 3.07-2.80 (m, 2H); MS (ESI+) m/z=299 (M+H).sup.+.
Example 123B
(R)-methyl 4-(7-hydroxy-4-(methoxyimino)chroman-2-yl)benzoate
[1882] The mixture of Example 123A (960 mg, 3.22 mmol), sodium acetate (528 mg, 6.44 mmol) and O-methylhydroxylamine, hydrochloric acid (538 mg, 6.44 mmol) in methanol (10 mL) was stirred at 60.degree. C. overnight. Solvent was removed under reduced pressure. The residue was dissolved in ethyl acetate and washed with water. The organic layers was dried over MgSO.sub.4, filtered, and concentrated. The residue was washed with ether to provide the title compound (810 mg, 2.475 mmol, 77% yield). .sup.1H NMR (400 MHz, CDCl.sub.3) .delta. 8.15-8.03 (m, 2H), 7.81 (d, J=8.7 Hz, 1H), 7.58-7.43 (m, 2H), 6.50 (dd, J=8.6, 2.5 Hz, 1H), 6.45 (d, J=2.5 Hz, 1H), 5.21 (d, J=3.0 Hz, 1H), 5.12 (dd, J=12.2, 3.2 Hz, 1H), 3.95 (s, 3H), 3.93 (s, 3H), 3.45 (dd, J=17.2, 3.2 Hz, 1H), 2.63 (dd, J=17.2, 12.2 Hz, 1H); MS (ESI+) m/z 328 (M+H).sup.+.
Example 123C
Methyl 4-((2R,4R)-4-amino-7-hydroxychroman-2-yl)benzoate
[1883] A mixture of Example 123B (570 mg, 1.741 mmol) was treated with 5% platinum (0.05 equivalent) on carbon in acetic acid (5 mL). The reaction was stirred at room temperature under hydrogen (1 atmosphere) for 24 hours, LC/MS showed conversion over 95%. The mixture was filtered through a celite pad and solvent removed under reduced pressure. The residue was purified by preparative LC method TFA2 to provide the trifluroroacetic acid salt of the title compound (300 mg, 44% yield). LC/MS m/z 283 (M-NH.sub.2).sup.+.
Example 123D
methyl 4-((2R,4R)-4-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanec- arboxamido)-7-hydroxychroman-2-yl)benzoate
[1884] A mixture of 1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxylic acid (162 mg, 0.668 mmol) and HATU (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate, 380 mg, 1.0 mmol) in DMF (2 mL) was stirred for 5 minutes at room temperature, followed by the addition of Example 123C (200 mg, 0.334 mmol) and N-ethyl-N-isopropylpropan-2-amine (0.466 ml, 2.67 mmol). The mixture was stirred at room temperature for 2 hours, LC/MS showed reaction complete. The mixture was loaded on to a 25 g silica gel cartridge eluting with 5-50% ethyl acetate in heptane provide the title compound (204 mg, 58.3% yield). .sup.1H NMR (400 MHz, CDCl.sub.3) .delta. 8.11-7.90 (m, 2H), 7.42 (d, J=8.0 Hz, 2H), 7.16-7.02 (m, 2H), 6.94 (dd, J=37.7, 8.3 Hz, 2H), 6.49-6.32 (m, 2H), 5.67 (s, 1H), 5.36 (dt, J=15.3, 8.7 Hz, 2H), 5.18 (d, J=10.7 Hz, 1H), 3.93 (s, 3H), 2.56-2.36 (m, 1H), 1.80-1.70 (m, 2H), 1.26 (d, J=2.2 Hz, 1H), 1.10-1.04 (m, 2H); MS (ESI-) m/z=521.9 (M-H).sup.-.
Example 123E
Methyl 4-((2R,4R)-4-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanec- arboxamido)-7-(difluoromethoxy)chroman-2-yl)benzoate
[1885] To Example 123D (190 mg, 0.363 mmol) and diethyl(bromodifluoromethyl)phosphonate (0.129 ml, 0.726 mmol) in a mixture of acetonitrile (2 mL) and water (1 mL) was added 50% aqueous potassium hydroxide (244 mg, 2.178 mmol) drop wise via syringe while stirring vigorously. After the addition was completed, LC/MS showed conversion was complete with a small by-product peak. Additional water was added to the mixture and the mixture was extracted with ethyl acetate (3.times.20 mL). The combined organic extracts were washed with 1 M HCl (5 mL) and water, dried over MgSO.sub.4, filtered, and concentrated. The residue was purified by preparative LC method TFA2 to provide the title compound (150 mg, 72% yield). .sup.1H NMR (400 MHz, CDCl.sub.3) .delta. 8.09-8.00 (m, 2H), 7.49-7.41 (m, 2H), 7.15-6.99 (m, 4H), 6.75-6.66 (m, 2H), 5.50-5.40 (m, 1H), 5.33 (d, J=8.9 Hz, 1H), 5.25 (dd, J=11.3, 2.0 Hz, 1H), 3.93 (s, 3H), 2.50 (ddd, J=13.4, 6.1, 2.1 Hz, 1H), 1.84-1.71 (m, 2H), 1.65 (d, J=2.8 Hz, 1H), 1.11-1.06 (m, 2H); MS (ESI-) m/z=572 (M-H).sup.-.
REFERENCE
Next up is Xueqing Wang of @abbvie speaking about a collaboration with @GalapagosNV on a different cystic fibrosis treatment #ACSSanFran
///////////ABBV 2222
O=C(O)c1ccc(cc1)[C@@H]3Oc2cc(OC(F)F)ccc2C(C3)NC(=O)C4(CC4)c5ccc6OC(F)(F)Oc6c5